Clinical trial outcomes for SLE: what we have and what we need
- PMID: 38360028
- PMCID: PMC10875561
- DOI: 10.1136/lupus-2023-001114
Clinical trial outcomes for SLE: what we have and what we need
Abstract
The paradigm of drug approval in SLE currently relies on successful large phase III randomised controlled trials and a set of primary, secondary and additional end points. Taken together, these outcomes offer a nuanced understanding of the efficacy and safety of the investigational agent. In this review, we thoroughly examine the main outcomes used in SLE trials and highlight unmet requirements as well as potential venues for future trial design in SLE. Disease activity indices can be broadly categorised into global-specific and organ-specific indices, in particular for skin, joints and kidneys, but there is no universal consensus about their use in clinical trials. Because each of these instruments has its own intrinsic strengths and weaknesses, the assessment of treatment response has progressed from relying solely on one individual disease activity index to using composite responder definitions. Those are typically measured from the trial baseline to the end point assessment date and may be combined with the need to taper and maintain glucocorticoids (GCs) within prespecified ranges. Remission and low disease activity are two critical states in the perspective of 'Treat-to-Target' trials, but are not fully recognised by regulators. While significant progress has been made in clinical trial outcomes for SLE, there is a clear need for continued innovation. Addressing these challenges will require collaboration between researchers, clinicians, patients as well as with regulatory agencies to refine existing outcome measures, incorporate meaningful and ethnically diverse patient perspectives, foster relevant digital opportunities and explore new therapeutic avenues, including early use of investigational agents. By doing so, we can advance our ability to manage SLE effectively and safely and improve the lives of those living with this complex and impactful autoimmune disease.
Keywords: autoimmune diseases; clinical trial; disease activity; systemic lupus erythematosus.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LA has received research funding and/or consulting honoraria from Alexion, Amgen, AstraZeneca, AbbVie, Alpine, Biogen, BMS, Boehringer-Ingelheim, Chugaï, GSK, Grifols, Janssen-Cilag, Kezar, LFB, Lilly, Medac, Novartis, Oséus, Pfizer, Roche, UCB. IP has received research funding and/or honoraria from Amgen, AstraZeneca, Aurinia Pharmaceuticals, Elli Lilly, Gilead, GSK, Janssen, Novartis, Otsuka and Roche. FC has received grant/research support from AstraZeneca, BMS and GSK; participated in an advisory board related to lupus for AstraZeneca, GSK, Celgene, Merck, horizon therapeutics and Principabio and received speaking fees and honoraria from AstraZeneca and GSK BMS related to lupus. HD has received consultant fees from GSK, Novartis, Janssen-Cilag and Axonal, and non-financial advantages from BMS, Novartis, Covantec, Teva Santé, LFB and Amgen.
Figures

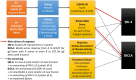
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Measuring outcomes in systemic lupus erythematosus clinical trials.Expert Rev Pharmacoecon Outcomes Res. 2011 Aug;11(4):455-68. doi: 10.1586/erp.11.38. Expert Rev Pharmacoecon Outcomes Res. 2011. PMID: 21831027 Review.
-
What Does It Mean to Be a British Isles Lupus Assessment Group-Based Composite Lupus Assessment Responder? Post Hoc Analysis of Two Phase III Trials.Arthritis Rheumatol. 2021 Nov;73(11):2059-2068. doi: 10.1002/art.41778. Epub 2021 Sep 22. Arthritis Rheumatol. 2021. PMID: 33913260 Free PMC article. Clinical Trial.
-
A critical review of clinical trials in systemic lupus erythematosus.Lupus. 2016 Sep;25(10):1122-40. doi: 10.1177/0961203316652492. Lupus. 2016. PMID: 27497257 Free PMC article. Review.
-
Clinical trial parameters that influence outcomes in lupus trials that use the systemic lupus erythematosus responder index.Rheumatology (Oxford). 2018 Jan 1;57(1):125-133. doi: 10.1093/rheumatology/kex368. Rheumatology (Oxford). 2018. PMID: 29045736
Cited by
-
Selenium: 48-year journey of global clinical trials.Mol Cell Biochem. 2025 Jun;480(6):3253-3265. doi: 10.1007/s11010-024-05202-x. Epub 2025 Jan 4. Mol Cell Biochem. 2025. PMID: 39755855 Review.
-
Development and evaluation of a Register-Based Organ Damage Index in systemic lupus erythematosus: a nationwide, population-based study from Sweden.Lupus Sci Med. 2025 Feb 26;12(1):e001403. doi: 10.1136/lupus-2024-001403. Lupus Sci Med. 2025. PMID: 40011068 Free PMC article.
-
Efficacy, pharmacokinetics and safety of iscalimab (CFZ533) in patients with proliferative lupus nephritis: a randomised, double-blind, placebo-controlled, phase II study.RMD Open. 2025 Aug 14;11(3):e005557. doi: 10.1136/rmdopen-2025-005557. RMD Open. 2025. PMID: 40813108 Free PMC article. Clinical Trial.
-
Mitochondrial Dysfunction in Systemic Lupus Erythematosus: Insights and Therapeutic Potential.Diseases. 2024 Sep 23;12(9):226. doi: 10.3390/diseases12090226. Diseases. 2024. PMID: 39329895 Free PMC article. Review.
References
-
- Guidance for industry: systemic lupus erythematosus--developing medical products for treatment. 2010.
-
- Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
