Long-term safety and effectiveness of canakinumab in patients with monogenic autoinflammatory diseases: results from the interim analysis of the RELIANCE registry
- PMID: 38360038
- PMCID: PMC10875478
- DOI: 10.1136/rmdopen-2023-003890
Long-term safety and effectiveness of canakinumab in patients with monogenic autoinflammatory diseases: results from the interim analysis of the RELIANCE registry
Abstract
Objective: Interim analysis of the RELIANCE registry, an on-going, non-interventional, open-label, multicentre, prospective study evaluating the long-term safety, dosing regimens and effectiveness of canakinumab in patients with cryopyrin-associated periodic syndromes (CAPS), familial Mediterranean fever (FMF), tumour-necrosis factor receptor-associated periodic syndrome (TRAPS) or mevalonate-kinase deficiency (MKD)/hyperimmunoglobulin-D syndrome (HIDS).
Methods: From September 2017 for patients with CAPS, and June 2018 for patients with FMF, TRAPS or MKD/HIDS, the registry enrolled paediatric (aged ≥2 years) and adult patients (aged ≥18 years) receiving canakinumab as part of their routine medical care. Safety, canakinumab dose, disease activity and quality of life outcome measures were evaluated at baseline and every 6 months until end of study visit.
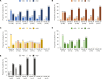
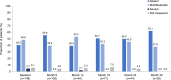
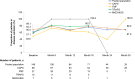
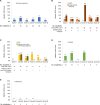
Results: At the analysis cut-off date (December 2020), 168 patients (91 CAPS, 54 FMF, 16 TRAPS and 7 MKD/HIDS) were enrolled. 85 (50.9%) patients were female and 72 (43.1%) were children (<18 years). The median patient age was 20.0 years (range 2.0-79.0 years). In the CAPS cohort, serious infections and serious adverse drug-reactions were more common in patients receiving higher than the recommended starting dose (SD) of canakinumab. A trend to receive >SD of canakinumab was observed in the pooled population. The majority of patients were reported as having either absent or mild/moderate disease activity (physician's global assessment) from baseline to Month 30, with a stable proportion of patients (~70%) in remission under canakinumab treatment. Patient-reported disease activity (Visual Analogue Scale (VAS), Autoinflammatory Disease Activity Index), fatigue (VAS); markers of inflammation (C-reactive protein, serum amyloid A and erythrocyte sedimentation rate) remained well-controlled throughout.
Conclusion: Data from this analysis confirm the long-term safety and effectiveness of canakinumab for the treatment of CAPS, FMF, TRAPS and MKD/HIDS.
Keywords: Cryopyrin-Associated Periodic Syndromes; Familial Mediterranean Fever; Inflammation.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JBK-D has received grant/research support from Novartis, AbbVie, Sobi and is a consultant of Novartis, AbbVie, Sobi. TKa received research support from Novartis. JH has received grant/research support from Novartis, Roche, SOBI and is a consultant of Novartis and has contributed to a speakers bureau with AbbVie, AstraZeneca, BMS, Boehringer-Ingelheim, Chugai, Janssen, Novartis, Pfizer, GSK, Sobi, Roche and UCB. BK-G is a consultant of Novartis. PTO has received study support from Novartis. JR has received grants from Novartis and Sobi; speaker fees from AbbVie, Biogen, BMS, Chugai, GSK, Janssen, Lilly, MSD, Mylan, Novartis, Roche, Sanofi, Sobi and UCB; and consultancy for AbbVie, Biogen, BMS, Chugai, GSK, Janssen, Lilly, MSD, Mylan, Novartis, Roche, Sanofi, Sobi and UCB. FW-H has no disclosures. GH has received grant/research support from AbbVie, Chugai, Merck Sharp & Dohme, Novartis, Pfizer and Roche and contributed to speakers bureau with AbbVie, Bayer, Chugai, Merck Sharp & Dohme, Novartis, Pfizer and Roche. AJ has received study support from Novartis. IF is a consultant of Novartis. CS has received study support from Novartis. FD has received study support from Novartis and is a consultant of AbbVie, Mylan, Novartis and Pfizer. MB has received grant/research support from Pfizer and Shire. MH has received study support from Novartis. FM has received honoraria from Novartis. MF has received support from AbbVie, Novartis, Pfizer, Galapagos and Amgen. TK has received study support, speaker fees and consultancy fees from Novartis. IA has contributed to a speakers bureau with AbbVie, Chugai, Novartis, UCB, MSD, Lilly, Sobi, AstraZeneca, Amgen, Pfizer and Gilead; received consultant fees from AstraZeneca and UCB; is a consultant for AbbVie, Chugai, Novartis, UCB, Galapagos, Takeda, AstraZeneca, Lilly, Boehringer Ingelheim, Amgen and Sobi. JW-A is an employee of Novartis. NB has received grant/research support from Novartis and Sobi; is a consultant of Novartis, Sobi, Lilly, Pfizer, AbbVie, BMS, MSD, Actelion, UCB, Boehringer-Ingelheim and Roche.
Figures

Similar articles
-
Long-Term Safety and Effectiveness of Canakinumab in Patients with MKD/HIDS: Interim Analysis of the RELIANCE Registry.Rheumatol Ther. 2025 Feb;12(1):137-155. doi: 10.1007/s40744-024-00733-7. Epub 2024 Dec 26. Rheumatol Ther. 2025. PMID: 39724475 Free PMC article.
-
Reasons for canakinumab initiation among patients with periodic fever syndromes: a retrospective medical chart review from the United States.Pediatr Rheumatol Online J. 2021 Sep 14;19(1):143. doi: 10.1186/s12969-021-00605-2. Pediatr Rheumatol Online J. 2021. PMID: 34521444 Free PMC article.
-
Systematic literature review of efficacy/effectiveness and safety of current therapies for the treatment of cryopyrin-associated periodic syndrome, hyperimmunoglobulin D syndrome and tumour necrosis factor receptor-associated periodic syndrome.RMD Open. 2020 Jul;6(2):e001227. doi: 10.1136/rmdopen-2020-001227. RMD Open. 2020. PMID: 32723831 Free PMC article.
-
Canakinumab treatment real world evidence in 3 monogenic periodic fever syndromes in 2009-2022: an interim analysis using the French JIR cohort database.Arthritis Res Ther. 2024 Apr 8;26(1):80. doi: 10.1186/s13075-024-03316-7. Arthritis Res Ther. 2024. PMID: 38589954 Free PMC article.
-
Consensus protocols for the diagnosis and management of the hereditary autoinflammatory syndromes CAPS, TRAPS and MKD/HIDS: a German PRO-KIND initiative.Pediatr Rheumatol Online J. 2020 Feb 17;18(1):17. doi: 10.1186/s12969-020-0409-3. Pediatr Rheumatol Online J. 2020. PMID: 32066461 Free PMC article.
Cited by
-
The past 25 years in paediatric rheumatology: insights from monogenic diseases.Nat Rev Rheumatol. 2024 Sep;20(9):585-593. doi: 10.1038/s41584-024-01145-1. Epub 2024 Aug 7. Nat Rev Rheumatol. 2024. PMID: 39112602 Review.
-
Canakinumab treatment patterns in sJIA, FMF, TRAPS, and MKD/HIDS: real-world insights from a Belgian non-interventional study.BMC Rheumatol. 2025 May 29;9(1):64. doi: 10.1186/s41927-025-00515-w. BMC Rheumatol. 2025. PMID: 40442768 Free PMC article.
-
Long-Term Safety and Effectiveness of Canakinumab in Patients with MKD/HIDS: Interim Analysis of the RELIANCE Registry.Rheumatol Ther. 2025 Feb;12(1):137-155. doi: 10.1007/s40744-024-00733-7. Epub 2024 Dec 26. Rheumatol Ther. 2025. PMID: 39724475 Free PMC article.
-
Tocilizumab effectively reduces flares of hyperimmunoglobulin D syndrome in children: Three cases in China.Mol Genet Metab Rep. 2024 Jun 17;40:101105. doi: 10.1016/j.ymgmr.2024.101105. eCollection 2024 Sep. Mol Genet Metab Rep. 2024. PMID: 38983106 Free PMC article.
-
Post-Marketing Pharmacovigilance of Canakinumab from the FDA Adverse Event Reporting System (FAERS).Pharmaceuticals (Basel). 2025 Jan 16;18(1):114. doi: 10.3390/ph18010114. Pharmaceuticals (Basel). 2025. PMID: 39861175 Free PMC article.
References
-
- Romano M, Arici ZS, Piskin D, et al. . The 2021 EULAR/American College of Rheumatology points to consider for diagnosis, management and monitoring of the interleukin-1 mediated autoinflammatory diseases: cryopyrin-associated periodic syndromes, tumour necrosis factor receptor-associated periodic syndrome, mevalonate kinase deficiency, and deficiency of the interleukin-1 receptor antagonist. Arthritis Rheumatol 2022;74:1102–21. 10.1002/art.42139 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
