Association of nociplastic and neuropathic pain components with the presence of residual symptoms in patients with axial spondyloarthritis receiving biological disease-modifying antirheumatic drugs
- PMID: 38360039
- PMCID: PMC10875534
- DOI: 10.1136/rmdopen-2023-004009
Association of nociplastic and neuropathic pain components with the presence of residual symptoms in patients with axial spondyloarthritis receiving biological disease-modifying antirheumatic drugs
Erratum in
-
Correction: Association of nociplastic and neuropathic pain components with the presence of residual symptoms in patients with axial spondyloarthritis receiving biological disease-modifying antirheumatic drugs.RMD Open. 2024 Apr 4;10(2):e004009corr1. doi: 10.1136/rmdopen-2023-004009corr1. RMD Open. 2024. PMID: 38580352 Free PMC article. No abstract available.
Abstract
Objective: To evaluate the association of nociplastic (NoP) and neuropathic pain (NP) components with residual symptoms in patients with radiographic axial spondyloarthritis (r-axSpA) receiving biological disease-modifying antirheumatic drugs (bDMARDs).
Methods: 78 patients with r-axSpA from the GErman SPondyloarthritis Inception Cohort receiving a bDMARD for at least 3 months were included in this analysis. The Widespread Pain Index (WPI) and the PainDETECT (PD) questionnaire were used to quantify the NoP and the NP components, respectively. Axial Spondyloarthritis Disease Activity Score (ASDAS) and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) were used as measures of residual symptoms. C reactive protein (CRP) was used as a measure of systemic inflammatory activity. Univariable and multivariable regression analyses of disease activity were performed. The regions of the WPI score and items of the PD score were used for cluster analyses.
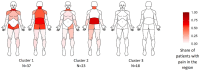
Results: Linear multivariable regression analysis showed that WPI and PD were independently associated with ASDAS (b=0.1, 95% CI 0.04 to 0.17, and b=0.05, 95% CI 0.02 to 0.08, respectively) and BASDAI (b=0.24, 95% CI 0.08 to 0.39, and b=0.17, 95% CI 0.1 to 0.25, respectively) in r-axSpA patients receiving stable treatment with bDMARDs. Furthermore, WPI and PD were found to be significantly associated with the presence of relevant residual symptoms as defined by BASDAI ≥4 (OR 1.93, 95% CI 1.09 to 4.15, and OR 1.32, 95% CI 1.04 to 1.85, respectively). The effects were present also in patients with normal level of CRP. Cluster analysis revealed three distinct pain distribution profiles and four specific sensory symptom constellations allowing differentiation of different pain subtypes.
Conclusion: Both NoP and NP components seem to be associated with residual symptoms in patients with r-axSpA receiving treatment with bDMARDs.
Keywords: Ankylosing Spondylitis; Disease Activity; Pain; Spondyloarthritis; Tumor Necrosis Factor Inhibitors.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Figures
Similar articles
-
Eligibility criteria for biologic disease-modifying antirheumatic drugs in axial spondyloarthritis: going beyond BASDAI.RMD Open. 2020 Jan;6(1):e001145. doi: 10.1136/rmdopen-2019-001145. RMD Open. 2020. PMID: 32144137 Free PMC article.
-
Achievement rate and predictive factors of the recommended therapeutical target in patients with axial spondyloarthritis who remain on biological therapy: a prospective cohort study in Spain.BMJ Open. 2022 Apr 29;12(4):e057850. doi: 10.1136/bmjopen-2021-057850. BMJ Open. 2022. PMID: 35487753 Free PMC article.
-
Validity and reliability of the Ankylosing Spondylitis Disease Activity Score with C-reactive protein (ASDAS-CRP) and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) in patients with axial spondyloarthritis (axSpA) in Singapore.Int J Rheum Dis. 2019 Dec;22(12):2206-2212. doi: 10.1111/1756-185X.13735. Epub 2019 Nov 13. Int J Rheum Dis. 2019. PMID: 31721427
-
Comparison of Ankylosing Spondylitis Disease Activity Score and Bath Ankylosing Spondylitis Disease Activity Index tools in assessment of axial spondyloarthritis activity.Reumatologia. 2024;62(1):64-69. doi: 10.5114/reum/185429. Epub 2024 Mar 18. Reumatologia. 2024. PMID: 38558891 Free PMC article. Review.
-
How to Monitor Disease Activity of Axial Spondyloarthritis in Clinical Practice.Curr Rheumatol Rep. 2024 May;26(5):170-177. doi: 10.1007/s11926-024-01141-0. Epub 2024 Feb 19. Curr Rheumatol Rep. 2024. PMID: 38372873 Review.
Cited by
-
[Chronic pain syndrome in musculoskeletal diseases-how different are fibromyalgia and long Covid?-Part 1].Z Rheumatol. 2025 May;84(4):312-319. doi: 10.1007/s00393-024-01603-x. Epub 2025 Jan 31. Z Rheumatol. 2025. PMID: 39888378 Review. German.
-
Difficult-to-Treat Axial Spondyloarthritis: A New Challenge.Drugs. 2024 Dec;84(12):1501-1508. doi: 10.1007/s40265-024-02100-w. Epub 2024 Oct 10. Drugs. 2024. PMID: 39388075 Review.
-
Understanding the causes of treatment failure is crucial for the management of axial spondyloarthritis.Nat Rev Rheumatol. 2025 Jun;21(6):309-311. doi: 10.1038/s41584-025-01254-5. Nat Rev Rheumatol. 2025. PMID: 40269319 No abstract available.
-
Psychosocial burden of axial spondyloarthritis and impact of different disease domains: a systematic literature review.Rheumatol Adv Pract. 2025 May 26;9(3):rkaf063. doi: 10.1093/rap/rkaf063. eCollection 2025. Rheumatol Adv Pract. 2025. PMID: 40584930 Free PMC article. Review.
-
Chronic Pain and Bone-Related Pathologies: A Narrative Review.J Pain Res. 2024 Sep 5;17:2937-2947. doi: 10.2147/JPR.S469229. eCollection 2024. J Pain Res. 2024. PMID: 39253740 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous