Repetitive transcranial magnetic stimulation as a treatment for Alzheimer's disease: A randomized placebo-controlled double-blind clinical trial
- PMID: 38360452
- PMCID: PMC10937236
- DOI: 10.1016/j.neurot.2024.e00331
Repetitive transcranial magnetic stimulation as a treatment for Alzheimer's disease: A randomized placebo-controlled double-blind clinical trial
Erratum in
-
Corrigendum to "Repetitive transcranial magnetic stimulation as a treatment for Alzheimer's disease: A randomized placebo-controlled double-blind clinical trial" [Neurotherapeutics 21 (3) (2024) e00331].Neurotherapeutics. 2024 Sep;21(5):100438. doi: 10.1016/j.neurot.2024.100438. Epub 2024 Aug 30. Neurotherapeutics. 2024. PMID: 39214819 Free PMC article. No abstract available.
Abstract
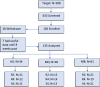
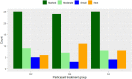
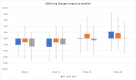
We report results of a large multisite double-blind randomized trial investigating the short and long-term efficacy of repetitive transcranial magnetic stimulation (rTMS) applied to patients with Alzheimer's disease (AD) at mild to moderate stages, in doses of either 2 or 4 weeks of treatment (5 days/week), whilst compared with 4 weeks of sham rTMS. Randomization to treatment group was stratified based on age and severity. The objectives of this study were to: 1) investigate the efficacy of active rTMS versus sham, 2) investigate the effect of dose of treatment (2 or 4 weeks), and 3) investigate the length of benefits from treatment. The rTMS pulses (20 Hz, 30 pulses/train, 25 trains, 10-s intertrain interval) were applied serially to the left and right dorsolateral prefrontal cortex using neuro-navigation. We compared the primary outcome measure's (ADAS-Cog) score changes from pre- to post-treatment, with assessments at baseline and 4 more times up to 6 months post-treatment. Data of 135 patients were analyzed. The mean total ADAS-Cog score at baseline did not differ between the active and sham treatment groups, nor across the three study sites. The overall results show significant cognitive improvement after treatment up to two months post-treatment with either sham or active coils. The results show both short and long-term benefits of active rTMS treatment but also show similar benefits for sham coil treatment of mild/moderate AD. We discuss this finding in the context of the existing literature on rTMS therapy for AD, as well as evidence of the sham coil's potential to induce a low-level current in the brain. TRIAL REGISTRATION: https://clinicaltrials.gov/ct2/show/NCT02908815.
Keywords: Alzheimer's disease; Dorsolateral prefrontal cortex; Double-blind; Randomized clinical trial; rTMS efficacy.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Zahra Moussavi reports financial support was provided by Weston Brain Institute. Paul B Fitzgerald reports a relationship with National Health and Medical Research Council of Australia Investigator grant (1193596) that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Cotelli M., Manenti R., Cappa S.F., Zanetti O., Miniussi C. Improved language performance in Alzheimer disease following brain stimulation. J Neurol Neurosurg Psychiatry. 2010;82:794–797. - PubMed
-
- Li X., Qi G., Yu C., Lia G., Zheng H., Wu S., et al. Cortical plasticity is correlated with cognitive improvement in Alzheimer’s disease patients after rTMS treatment. Brain Stimul. 2021;14(3):503–510. - PubMed
-
- Zhang F., Qin Y., Xie L., Zheng C., Huang X., Zhang M. High-frequency repetitive transcranial magnetic stimulation combined with cognitive training improves cognitive function and cortical metabolic ratios in Alzheimer’s disease. J Neural Transm. 2019;126(8):1081–1094. - PubMed
-
- Sabbagh M., Sadowsky C., Tousi B., Agronin M.E., Alva G., Armon C., et al. Effects of a combined transcranial magnetic stimulation (TMS) and cognitive training intervention in patients with Alzheimer’s disease. Alzheimer’s Dementia. 2019;16(4):641–650. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

