Identification of CD160-TM as a tumor target on triple negative breast cancers: possible therapeutic applications
- PMID: 38360636
- PMCID: PMC10870674
- DOI: 10.1186/s13058-024-01785-x
Identification of CD160-TM as a tumor target on triple negative breast cancers: possible therapeutic applications
Abstract
Background: Despite major therapeutic advances, triple-negative breast cancer (TNBC) still presents a worth prognosis than hormone receptors-positive breast cancers. One major issue relies in the molecular and mutational heterogeneity of TNBC subtypes that is reinforced by the absence of reliable tumor-antigen that could serve as a specific target to further promote efficient tumor cell recognition and depletion. CD160 is a receptor mainly expressed by NK lymphocytes and presenting two isoforms, namely the GPI-anchored form (CD160-GPI) and the transmembrane isoform (CD160-TM). While CD160-GPI is constitutively expressed on resting cells and involved in the generation of NK cells' cytotoxic activity, CD160-TM is neo-synthesized upon activation and promotes the amplification of NK cells' killing ability.
Methods: CD160 expression was assessed by immunohistochemistry (IHC) and flow cytometry on TNBC patient biopsies or cell lines, respectively. Antibody (Ab)-mediated tumor depletion was tested in vitro by performing antibody-dependent cell cytotoxicity (ADCC) and phagocytosis (ADCP) assays, and in vivo on a TNBC mouse model.
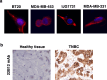
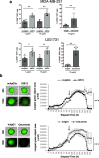
Results: Preliminary data obtained by IHC on TNBC patients' tumor biopsies revealed an unconventional expression of CD160 by TNBC tumor cells. By using a specific but conformation-dependent anti-CD160-TM Ab, we established that CD160-TM, but not CD160-GPI, was expressed by TNBC tumor cells. A conformation-independent anti-CD160-TM mAb (22B12; muIgG2a isotype) was generated and selected according to pre-defined specificity and functional criterions. In vitro functional assays demonstrated that ADCC and ADCP could be induced in the presence of 22B12, resulting in TNBC cell line apoptosis. The ability of 22B12 to exert an in vivo anti-tumor activity was also demonstrated on a TNBC murine model.
Conclusions: Our data identify CD160-TM as a tumor marker for TNBC and provide a rational for the use of anti-CD160-TM antibodies as therapeutic tools in this tumor context.
Keywords: Antibody-based therapy; CD160-TM; TNBC; Tumor antigen.
© 2024. The Author(s).
Conflict of interest statement
A.M-C, J.G and A.B are founding members of Alderaan Biotechnology. AF has ownership interest (including patents) in Alderaan Biotechnology. No conflicts of interest were disclosed by the other authors.
Figures



References
-
- Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

