Acute neutrophilic vasculitis (leukocytoclasia) in 36 COVID-19 autopsy brains
- PMID: 38360666
- PMCID: PMC10870569
- DOI: 10.1186/s13000-024-01445-w
Acute neutrophilic vasculitis (leukocytoclasia) in 36 COVID-19 autopsy brains
Abstract
Background: Hypercytokinemia, the renin-angiotensin system, hypoxia, immune dysregulation, and vasculopathy with evidence of immune-related damage are implicated in brain morbidity in COVID-19 along with a wide variety of genomic and environmental influences. There is relatively little evidence of direct SARS-CoV-2 brain infection in COVID-19 patients.
Methods: Brain histopathology of 36 consecutive autopsies of patients who were RT-PCR positive for SARS-CoV-2 was studied along with findings from contemporary and pre-pandemic historical control groups. Immunostaining for serum and blood cell proteins and for complement components was employed. Microcirculatory wall complement deposition in the COVID-19 cohort was compared to historical control cases. Comparisons also included other relevant clinicopathological and microcirculatory findings in the COVID-19 cohort and control groups.
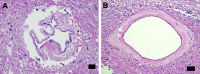
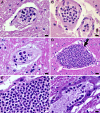
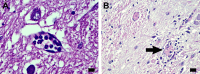
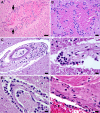
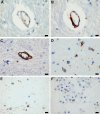
Results: The COVID-19 cohort and both the contemporary and historical control groups had the same rate of hypertension, diabetes mellitus, and obesity. The COVID-19 cohort had varying amounts of acute neutrophilic vasculitis with leukocytoclasia in the microcirculation of the brain in all cases. Prominent vascular neutrophilic transmural migration was found in several cases and 25 cases had acute perivasculitis. Paravascular microhemorrhages and petechial hemorrhages (small brain parenchymal hemorrhages) had a slight tendency to be more numerous in cohort cases that displayed less acute neutrophilic vasculitis. Tissue burden of acute neutrophilic vasculitis with leukocytoclasia was the same in control cases as a group, while it was significantly higher in COVID-19 cases. Both the tissue burden of acute neutrophilic vasculitis and the activation of complement components, including membrane attack complex, were significantly higher in microcirculatory channels in COVID-19 cohort brains than in historical controls.
Conclusions: Acute neutrophilic vasculitis with leukocytoclasia, acute perivasculitis, and associated paravascular blood extravasation into brain parenchyma constitute the first phase of an immune-related, acute small-vessel inflammatory condition often termed type 3 hypersensitivity vasculitis or leukocytoclastic vasculitis. There is a higher tissue burden of acute neutrophilic vasculitis and an increased level of activated complement components in microcirculatory walls in COVID-19 cases than in pre-pandemic control cases. These findings are consistent with a more extensive small-vessel immune-related vasculitis in COVID-19 cases than in control cases. The pathway(s) and mechanism for these findings are speculative.
Keywords: Acute neutrophilic vasculitis; Antigen-antibody complex; COVID-19; Central nervous system; Complement component; Karyorrhexis; Microcirculation; Microvessel; SARS-CoV-2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Leukocytoclastic vasculitis (cutaneous small-vessel vasculitis) after COVID-19 vaccination.J Autoimmun. 2022 Feb;127:102783. doi: 10.1016/j.jaut.2021.102783. Epub 2021 Dec 28. J Autoimmun. 2022. PMID: 34973526 Free PMC article. Review.
-
Cutaneous small-vessel vasculitis following COVID-19 vaccine.J Cosmet Dermatol. 2021 Nov;20(11):3382-3383. doi: 10.1111/jocd.14452. Epub 2021 Sep 16. J Cosmet Dermatol. 2021. PMID: 34529877 Free PMC article. No abstract available.
-
Cutaneous leukocytoclastic vasculitis secondary to COVID-19 infection leading to extensive skin necrosis.Clin Dermatol. 2022 Jul-Aug;40(4):397-401. doi: 10.1016/j.clindermatol.2022.02.013. Epub 2022 Mar 4. Clin Dermatol. 2022. PMID: 35248687 Free PMC article.
-
Leukocytoclastic vasculitis in association with linear epidermal basement membrane zone immunoglobulin deposition: Linear vasculitis.Clin Dermatol. 2022 Nov-Dec;40(6):639-650. doi: 10.1016/j.clindermatol.2022.07.011. Epub 2022 Jul 28. Clin Dermatol. 2022. PMID: 35907580
-
Cutaneous vasculitis update: small vessel neutrophilic vasculitis syndromes.Am J Dermatopathol. 2006 Dec;28(6):486-506. doi: 10.1097/01.dad.0000246646.45651.a2. Am J Dermatopathol. 2006. PMID: 17122493 Review.
Cited by
-
COVID-19 Cerebral Vasculitis With Extensive Involvement of Anterior Circulation and Sparing of Posterior Circulation.Cureus. 2025 Jun 26;17(6):e86818. doi: 10.7759/cureus.86818. eCollection 2025 Jun. Cureus. 2025. PMID: 40718331 Free PMC article.
-
Post-COVID dysphagia requires exclusion of SARS-CoV-2-associated brainstem encephalitis, vasculitis, polyneuritis cranialis, and myositis.Brain Behav. 2024 Sep;14(9):e70032. doi: 10.1002/brb3.70032. Brain Behav. 2024. PMID: 39317972 Free PMC article. No abstract available.
References
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

