Harnessing peroxisome proliferator-activated receptor γ agonists to induce Heme Oxygenase-1: a promising approach for pulmonary inflammatory disorders
- PMID: 38360670
- PMCID: PMC10868008
- DOI: 10.1186/s12964-024-01501-4
Harnessing peroxisome proliferator-activated receptor γ agonists to induce Heme Oxygenase-1: a promising approach for pulmonary inflammatory disorders
Abstract
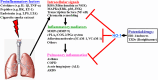
The activation of peroxisome proliferator-activated receptor (PPAR)-γ has been extensively shown to attenuate inflammatory responses in conditions such as asthma, acute lung injury, and acute respiratory distress syndrome, as demonstrated in animal studies. However, the precise molecular mechanisms underlying these inhibitory effects remain largely unknown. The upregulation of heme oxygenase-1 (HO-1) has been shown to confer protective effects, including antioxidant, antiapoptotic, and immunomodulatory effects in vitro and in vivo. PPARγ is highly expressed not only in adipose tissues but also in various other tissues, including the pulmonary system. Thiazolidinediones (TZDs) are highly selective agonists for PPARγ and are used as antihyperglycemic medications. These observations suggest that PPARγ agonists could modulate metabolism and inflammation. Several studies have indicated that PPARγ agonists may serve as potential therapeutic candidates in inflammation-related diseases by upregulating HO-1, which in turn modulates inflammatory responses. In the respiratory system, exposure to external insults triggers the expression of inflammatory molecules, such as cytokines, chemokines, adhesion molecules, matrix metalloproteinases, and reactive oxygen species, leading to the development of pulmonary inflammatory diseases. Previous studies have demonstrated that the upregulation of HO-1 protects tissues and cells from external insults, indicating that the induction of HO-1 by PPARγ agonists could exert protective effects by inhibiting inflammatory signaling pathways and attenuating the development of pulmonary inflammatory diseases. However, the mechanisms underlying TZD-induced HO-1 expression are not well understood. This review aimed to elucidate the molecular mechanisms through which PPARγ agonists induce the expression of HO-1 and explore how they protect against inflammatory and oxidative responses.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Haem oxygenase-1 up-regulation by rosiglitazone via ROS-dependent Nrf2-antioxidant response elements axis or PPARγ attenuates LPS-mediated lung inflammation.Br J Pharmacol. 2018 Oct;175(20):3928-3946. doi: 10.1111/bph.14465. Epub 2018 Sep 6. Br J Pharmacol. 2018. PMID: 30088830 Free PMC article.
-
Heme oxygenase-1 induction by rosiglitazone via PKCα/AMPKα/p38 MAPKα/SIRT1/PPARγ pathway suppresses lipopolysaccharide-mediated pulmonary inflammation.Biochem Pharmacol. 2018 Feb;148:222-237. doi: 10.1016/j.bcp.2017.12.024. Epub 2018 Jan 5. Biochem Pharmacol. 2018. PMID: 29309760
-
A novel hypothesis: up-regulation of HO-1 by activation of PPARγ inhibits HMGB1-RAGE signaling pathway and ameliorates the development of ALI/ARDS.J Thorac Dis. 2013 Oct;5(5):706-10. doi: 10.3978/j.issn.2072-1439.2013.08.69. J Thorac Dis. 2013. PMID: 24255785 Free PMC article.
-
Peroxisome proliferator-activated receptor gamma agonists as therapy for chronic airway inflammation.Eur J Pharmacol. 2006 Mar 8;533(1-3):101-9. doi: 10.1016/j.ejphar.2005.12.048. Epub 2006 Feb 3. Eur J Pharmacol. 2006. PMID: 16458290 Review.
-
PPAR-gamma in ulcerative colitis: a novel target for intervention.Curr Drug Targets. 2013 Nov;14(12):1501-7. doi: 10.2174/13894501113149990162. Curr Drug Targets. 2013. PMID: 23651165 Review.
Cited by
-
5-methoxytryptophan ameliorates renal ischemia/reperfusion injury by alleviating endoplasmic reticulum stress-mediated apoptosis through the Nrf2/HO-1 pathway.Front Pharmacol. 2025 Apr 14;16:1506482. doi: 10.3389/fphar.2025.1506482. eCollection 2025. Front Pharmacol. 2025. PMID: 40297140 Free PMC article.
-
Juglans regia L. (Walnut) Leaf Extract Ameliorates Pulmonary Edema Against Airway Inflammation via Upregulation of Tight Junction Proteins and Heme Oxygenase-1 in the Lungs of Asthmatic Mice.J Trop Med. 2025 May 19;2025:6976932. doi: 10.1155/jotm/6976932. eCollection 2025. J Trop Med. 2025. PMID: 40421046 Free PMC article.
-
The role of PPAR in fungal keratitis.Front Immunol. 2024 Dec 23;15:1454463. doi: 10.3389/fimmu.2024.1454463. eCollection 2024. Front Immunol. 2024. PMID: 39763659 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

