Targeting cancer-derived extracellular vesicles by combining CD147 inhibition with tissue factor pathway inhibitor for the management of urothelial cancer cells
- PMID: 38360687
- PMCID: PMC10870545
- DOI: 10.1186/s12964-024-01508-x
Targeting cancer-derived extracellular vesicles by combining CD147 inhibition with tissue factor pathway inhibitor for the management of urothelial cancer cells
Abstract
Background: Extracellular vesicles (EVs), including microvesicles, hold promise for the management of bladder urothelial carcinoma (BLCA), particularly because of their utility in identifying therapeutic targets and their diagnostic potential using easily accessible urine samples. Among the transmembrane glycoproteins highly enriched in cancer-derived EVs, tissue factor (TF) and CD147 have been implicated in promoting tumor progression. In this in vitro study, we explored a novel approach to impede cancer cell migration and metastasis by simultaneously targeting these molecules on urothelial cancer-derived EVs.
Methods: Cell culture supernatants from invasive and non-invasive bladder cancer cell lines and urine samples from patients with BLCA were collected. Large, microvesicle-like EVs were isolated using sequential centrifugation and characterized by electron microscopy, nanoparticle tracking analysis, and flow cytometry. The impact of urinary or cell supernatant-derived EVs on cellular phenotypes was evaluated using cell-based assays following combined treatment with a specific CD147 inhibitor alone or in combination with a tissue factor pathway inhibitor (TFPI), an endogenous anticoagulant protein that can be released by low-molecular-weight heparins.
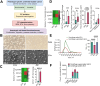
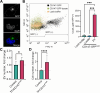
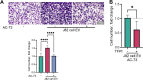
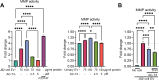
Results: We observed that EVs obtained from the urine samples of patients with muscle-invasive BLCA and from the aggressive bladder cancer cell line J82 exhibited higher TF activity and CD147 expression levels than did their non-invasive counterparts. The shedding of GFP-tagged CD147 into isolated vesicles demonstrated that the vesicles originated from plasma cell membranes. EVs originating from invasive cancer cells were found to trigger migration, secretion of matrix metalloproteinases (MMPs), and invasion. The same induction of MMP activity was replicated using EVs obtained from urine samples of patients with invasive BLCA. EVs derived from cancer cell clones overexpressing TF and CD147 were produced in higher quantities and exhibited a higher invasive potential than those from control cancer cells. TFPI interfered with the effect when used in conjunction with the CD147 inhibitor, further suppressing homotypic EV-induced migration, MMP production, and invasion.
Conclusions: Our findings suggest that combining a CD147 inhibitor with low molecular weight heparins to induce TFPI release may be a promising therapeutic approach for urothelial cancer management. This combination can potentially suppress the tumor-promoting actions of cancer-derived microvesicle-like EVs, including collective matrix invasion.
Keywords: Bladder carcinoma; CD142; EMMPRIN; Microvesicles.
Plain language summary
Small particles or vesicles released by cancer cells into their surroundings have the potential to stimulate the spread and growth of cancer cells. In this study, we focused on two specific molecules presented by these cancer cell-derived vesicles that could play a role in promoting the dissemination of cancer cells: a protein related to blood clotting and a protein on the cell surface.We found that large vesicles from bladder cancer cells that have the ability to spread had higher levels of these proteins than vesicles from nonspreading cancer cells. We also found that the former could make cancer cells move about more, produce more of a substance that helps cancer cells spread, and invade other tissues.To counteract the cancer-promoting actions of these vesicles, we examined the impact of combining a naturally occurring anticlotting protein that can be released by medications derived from heparin with an inhibitor targeting the cancer cell surface protein. We found that this combination stopped the vesicles from helping cancer cells move about more, produce more of the spreading substance, and invade other tissues.This approach of simultaneously targeting the two protein molecules present on cancer cell-derived vesicles might be a new way to treat bladder cancer.
© 2024. The Author(s).
Conflict of interest statement
This work was supported by the LEO Pharma Investigator-Initiated Studies fund. VKB received a scholarship from the LEO Pharma, and TS from the Ad Infinitum Foundation. The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

