Loss of macrophage TSC1 exacerbates sterile inflammatory liver injury through inhibiting the AKT/MST1/NRF2 signaling pathway
- PMID: 38360839
- PMCID: PMC10869801
- DOI: 10.1038/s41419-024-06538-4
Loss of macrophage TSC1 exacerbates sterile inflammatory liver injury through inhibiting the AKT/MST1/NRF2 signaling pathway
Abstract
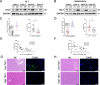
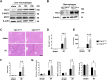
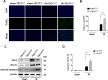
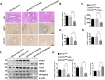
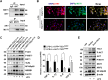
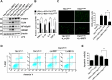
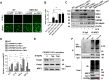
Tuberous sclerosis complex 1 (TSC1) plays important roles in regulating innate immunity. However, the precise role of TSC1 in macrophages in the regulation of oxidative stress response and hepatic inflammation in liver ischemia/reperfusion injury (I/R) remains unknown. In a mouse model of liver I/R injury, deletion of myeloid-specific TSC1 inhibited AKT and MST1 phosphorylation, and decreased NRF2 accumulation, whereas activated TLR4/NF-κB pathway, leading to increased hepatic inflammation. Adoptive transfer of AKT- or MST1-overexpressing macrophages, or Keap1 disruption in myeloid-specific TSC1-knockout mice promoted NRF2 activation but reduced TLR4 activity and mitigated I/R-induced liver inflammation. Mechanistically, TSC1 in macrophages promoted AKT and MST1 phosphorylation, and protected NRF2 from Keap1-mediated ubiquitination. Furthermore, overexpression AKT or MST1 in TSC1-knockout macrophages upregulated NRF2 expression, downregulated TLR4/NF-κB, resulting in reduced inflammatory factors, ROS and inflammatory cytokine-mediated hepatocyte apoptosis. Strikingly, TSC1 induction in NRF2-deficient macrophages failed to reverse the TLR4/NF-κB activity and production of pro-inflammatory factors. Conclusions: Macrophage TSC1 promoted the activation of the AKT/MST1 signaling pathway, increased NRF2 levels via reducing Keap1-mediated ubiquitination, and modulated oxidative stress-driven inflammatory responses in liver I/R injury. Our findings underscore the critical role of macrophage TSC1 as a novel regulator of innate immunity and imply the therapeutic potential for the treatment of sterile liver inflammation in transplant recipients. Schematic illustration of macrophage TSC1-mediated AKT/MST1/NRF2 signaling pathway in I/R-triggered liver inflammation. Macrophage TSC1 can be activated in I/R-stressed livers. TSC1 activation promotes phosphorylation of AKT and MST1, which in turn increases NRF2 expression and inhibits ROS production and TLR4/NF-κB activation, resulting in reduced hepatocellular apoptosis in I/R-triggered liver injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

