Quantitative systems pharmacology modeling of HER2-positive metastatic breast cancer for translational efficacy evaluation and combination assessment across therapeutic modalities
- PMID: 38360930
- PMCID: PMC11130324
- DOI: 10.1038/s41401-024-01232-9
Quantitative systems pharmacology modeling of HER2-positive metastatic breast cancer for translational efficacy evaluation and combination assessment across therapeutic modalities
Abstract
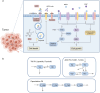
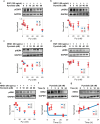
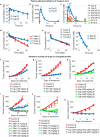
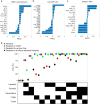
HER2-positive (HER2+) metastatic breast cancer (mBC) is highly aggressive and a major threat to human health. Despite the significant improvement in patients' prognosis given the drug development efforts during the past several decades, many clinical questions still remain to be addressed such as efficacy when combining different therapeutic modalities, best treatment sequences, interindividual variability as well as resistance and potential coping strategies. To better answer these questions, we developed a mechanistic quantitative systems pharmacology model of the pathophysiology of HER2+ mBC that was extensively calibrated and validated against multiscale data to quantitatively predict and characterize the signal transduction and preclinical tumor growth kinetics under different therapeutic interventions. Focusing on the second-line treatment for HER2+ mBC, e.g., antibody-drug conjugates (ADC), small molecule inhibitors/TKI and chemotherapy, the model accurately predicted the efficacy of various drug combinations and dosing regimens at the in vitro and in vivo levels. Sensitivity analyses and subsequent heterogeneous phenotype simulations revealed important insights into the design of new drug combinations to effectively overcome various resistance scenarios in HER2+ mBC treatments. In addition, the model predicted a better efficacy of the new TKI plus ADC combination which can potentially reduce drug dosage and toxicity, while it also shed light on the optimal treatment ordering of ADC versus TKI plus capecitabine regimens, and these findings were validated by new in vivo experiments. Our model is the first that mechanistically integrates multiple key drug modalities in HER2+ mBC research and it can serve as a high-throughput computational platform to guide future model-informed drug development and clinical translation.
Keywords: HER2+ metastatic breast cancer; drug resistance; model-informed drug development; quantitative systems pharmacology; therapeutic combinations.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Hurvitz SA, Hegg R, Chung WP, Im SA, Jacot W, Ganju V, et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet. 2023;401:105–17. doi: 10.1016/S0140-6736(22)02420-5. - DOI - PubMed
-
- Dieras V, Miles D, Verma S, Pegram M, Welslau M, Baselga J, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILIA): a descriptive analysis of final overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–42. doi: 10.1016/S1470-2045(17)30312-1. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

