Adipocyte p53 coordinates the response to intermittent fasting by regulating adipose tissue immune cell landscape
- PMID: 38360943
- PMCID: PMC10869344
- DOI: 10.1038/s41467-024-45724-y
Adipocyte p53 coordinates the response to intermittent fasting by regulating adipose tissue immune cell landscape
Abstract
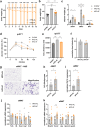
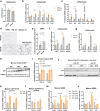
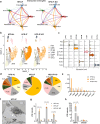
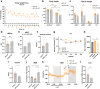
In obesity, sustained adipose tissue (AT) inflammation constitutes a cellular memory that limits the effectiveness of weight loss interventions. Yet, the impact of fasting regimens on the regulation of AT immune infiltration is still elusive. Here we show that intermittent fasting (IF) exacerbates the lipid-associated macrophage (LAM) inflammatory phenotype of visceral AT in obese mice. Importantly, this increase in LAM abundance is strongly p53 dependent and partly mediated by p53-driven adipocyte apoptosis. Adipocyte-specific deletion of p53 prevents LAM accumulation during IF, increases the catabolic state of adipocytes, and enhances systemic metabolic flexibility and insulin sensitivity. Finally, in cohorts of obese/diabetic patients, we describe a p53 polymorphism that links to efficacy of a fasting-mimicking diet and that the expression of p53 and TREM2 in AT negatively correlates with maintaining weight loss after bariatric surgery. Overall, our results demonstrate that p53 signalling in adipocytes dictates LAM accumulation in AT under IF and modulates fasting effectiveness in mice and humans.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Mechanisms of Lipid-Associated Macrophage Accrual in Metabolically Stressed Adipose Tissue.Bioessays. 2025 Apr;47(4):e202400203. doi: 10.1002/bies.202400203. Epub 2025 Jan 19. Bioessays. 2025. PMID: 39828607 Free PMC article. Review.
-
Inflammation and insulin resistance exert dual effects on adipose tissue tumor protein 53 expression.Int J Obes (Lond). 2014 May;38(5):737-45. doi: 10.1038/ijo.2013.163. Epub 2013 Sep 3. Int J Obes (Lond). 2014. PMID: 23999197
-
Impact of Reduced ATGL-Mediated Adipocyte Lipolysis on Obesity-Associated Insulin Resistance and Inflammation in Male Mice.Endocrinology. 2015 Oct;156(10):3610-24. doi: 10.1210/en.2015-1322. Epub 2015 Jul 21. Endocrinology. 2015. PMID: 26196542 Free PMC article.
-
p53 Functions in Adipose Tissue Metabolism and Homeostasis.Int J Mol Sci. 2018 Sep 4;19(9):2622. doi: 10.3390/ijms19092622. Int J Mol Sci. 2018. PMID: 30181511 Free PMC article. Review.
-
The multifunctional protein E4F1 links P53 to lipid metabolism in adipocytes.Nat Commun. 2021 Dec 2;12(1):7037. doi: 10.1038/s41467-021-27307-3. Nat Commun. 2021. PMID: 34857760 Free PMC article.
Cited by
-
Innate immune cells link dietary cues to normal and abnormal metabolic regulation.Nat Immunol. 2025 Jan;26(1):29-41. doi: 10.1038/s41590-024-02037-y. Epub 2025 Jan 2. Nat Immunol. 2025. PMID: 39747429 Free PMC article. Review.
-
Time-restricted feeding improves metabolic syndrome by activating thermogenesis in brown adipose tissue and reducing inflammatory markers.Front Immunol. 2025 Jan 24;16:1501850. doi: 10.3389/fimmu.2025.1501850. eCollection 2025. Front Immunol. 2025. PMID: 39925816 Free PMC article.
-
Tissue macrophages: origin, heterogenity, biological functions, diseases and therapeutic targets.Signal Transduct Target Ther. 2025 Mar 7;10(1):93. doi: 10.1038/s41392-025-02124-y. Signal Transduct Target Ther. 2025. PMID: 40055311 Free PMC article. Review.
-
Thermal stress, p53 structures and learning from elephants.Cell Death Discov. 2024 Aug 7;10(1):353. doi: 10.1038/s41420-024-02109-w. Cell Death Discov. 2024. PMID: 39107279 Free PMC article. Review.
-
Cellular Senescence and Extracellular Vesicles in the Pathogenesis and Treatment of Obesity-A Narrative Review.Int J Mol Sci. 2024 Jul 20;25(14):7943. doi: 10.3390/ijms25147943. Int J Mol Sci. 2024. PMID: 39063184 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- P 29328/FWF_/Austrian Science Fund FWF/Austria
- P 32400/FWF_/Austrian Science Fund FWF/Austria
- P34109/Austrian Science Fund (Fonds zur Förderung der Wissenschaftlichen Forschung)
- P 34109/FWF_/Austrian Science Fund FWF/Austria
- I3165/Austrian Science Fund (Fonds zur Förderung der Wissenschaftlichen Forschung)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

