Compressive stress triggers fibroblasts spreading over cancer cells to generate carcinoma in situ organization
- PMID: 38360973
- PMCID: PMC10869726
- DOI: 10.1038/s42003-024-05883-6
Compressive stress triggers fibroblasts spreading over cancer cells to generate carcinoma in situ organization
Abstract
At the early stage of tumor progression, fibroblasts are located at the outer edges of the tumor, forming an encasing layer around it. In this work, we have developed a 3D in vitro model where fibroblasts' layout resembles the structure seen in carcinoma in situ. We use a microfluidic encapsulation technology to co-culture fibroblasts and cancer cells within hollow, permeable, and elastic alginate shells. We find that in the absence of spatial constraint, fibroblasts and cancer cells do not mix but segregate into distinct aggregates composed of individual cell types. However, upon confinement, fibroblasts enwrap cancer cell spheroid. Using a combination of biophysical methods and live imaging, we find that buildup of compressive stress is required to induce fibroblasts spreading over the aggregates of tumor cells. We propose that compressive stress generated by the tumor growth might be a mechanism that prompts fibroblasts to form a capsule around the tumor.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
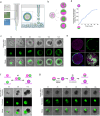

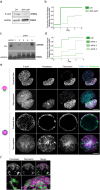


Similar articles
-
Controlling Cell Organization in 3D Coculture Spheroids Using DNA Interactions.ACS Biomater Sci Eng. 2023 Jun 12;9(6):3185-3192. doi: 10.1021/acsbiomaterials.3c00546. Epub 2023 May 8. ACS Biomater Sci Eng. 2023. PMID: 37155244
-
Characterization of 3D NSCLC Cell Cultures with Fibroblasts or Macrophages for Tumor Microenvironment Studies and Chemotherapy Screening.Cells. 2023 Dec 8;12(24):2790. doi: 10.3390/cells12242790. Cells. 2023. PMID: 38132110 Free PMC article.
-
Multicellular spheroid based on a triple co-culture: A novel 3D model to mimic pancreatic tumor complexity.Acta Biomater. 2018 Sep 15;78:296-307. doi: 10.1016/j.actbio.2018.08.008. Epub 2018 Aug 10. Acta Biomater. 2018. PMID: 30099198
-
[3D Tumor organoid models produced by cellular capsules technology CCT].Bull Cancer. 2022 Jan;109(1):38-48. doi: 10.1016/j.bulcan.2021.12.001. Epub 2022 Jan 5. Bull Cancer. 2022. PMID: 34996600 Review. French.
-
Heterotypic tumor spheroids: a platform for nanomedicine evaluation.J Nanobiotechnology. 2023 Aug 2;21(1):249. doi: 10.1186/s12951-023-02021-y. J Nanobiotechnology. 2023. PMID: 37533100 Free PMC article. Review.
Cited by
-
Instant fluorescence lifetime imaging microscopy reveals mechano-metabolic reprogramming of stromal cells in breast cancer peritumoral microenvironments.bioRxiv [Preprint]. 2025 May 30:2025.05.28.656717. doi: 10.1101/2025.05.28.656717. bioRxiv. 2025. PMID: 40501613 Free PMC article. Preprint.
-
In situ tailored confining microenvironment for lung cancer spheroids.Mater Today Bio. 2025 Feb 19;31:101602. doi: 10.1016/j.mtbio.2025.101602. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40070872 Free PMC article.
-
Compressive stresses in cancer: characterization and implications for tumour progression and treatment.Nat Rev Cancer. 2024 Nov;24(11):768-791. doi: 10.1038/s41568-024-00745-z. Epub 2024 Oct 10. Nat Rev Cancer. 2024. PMID: 39390249 Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

