Targeting NUPR1-dependent stress granules formation to induce synthetic lethality in KrasG12D-driven tumors
- PMID: 38360999
- PMCID: PMC10940650
- DOI: 10.1038/s44321-024-00032-2
Targeting NUPR1-dependent stress granules formation to induce synthetic lethality in KrasG12D-driven tumors
Abstract
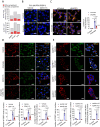
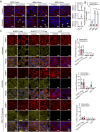
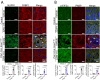
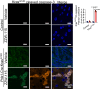
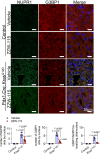
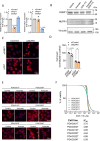
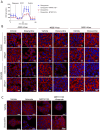
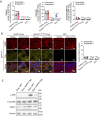
We find that NUPR1, a stress-associated intrinsically disordered protein, induced droplet formation via liquid-liquid phase separation (LLPS). NUPR1-driven LLPS was crucial for the creation of NUPR1-dependent stress granules (SGs) in pancreatic cancer cells since genetic or pharmacological inhibition by ZZW-115 of NUPR1 activity impeded SGs formation. The KrasG12D mutation induced oncogenic stress, NUPR1 overexpression, and promoted SGs development. Notably, enforced NUPR1 expression induced SGs formation independently of mutated KrasG12D. Mechanistically, KrasG12D expression strengthened sensitivity to NUPR1 inactivation, inducing cell death, activating caspase 3 and releasing LDH. Remarkably, ZZW-115-mediated SG-formation inhibition hampered the development of pancreatic intraepithelial neoplasia (PanINs) in Pdx1-cre;LSL-KrasG12D (KC) mice. ZZW-115-treatment of KC mice triggered caspase 3 activation, DNA fragmentation, and formation of the apoptotic bodies, leading to cell death, specifically in KrasG12D-expressing cells. We further demonstrated that, in developed PanINs, short-term ZZW-115 treatment prevented NUPR1-associated SGs presence. Lastly, a four-week ZZW-115 treatment significantly reduced the number and size of PanINs in KC mice. This study proposes that targeting NUPR1-dependent SGs formation could be a therapeutic approach to induce cell death in KrasG12D-dependent tumors.
Keywords: Kras; NUPR1; Stress Granules; Synthetic Lethality; ZZW-115.
© 2024. The Author(s).
Conflict of interest statement
JI is co-founder of PanCa Therapeutics and PredictingMed. The other authors declare no competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures





References
-
- Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M. Formation of stress granules inhibits apoptosis by suppressing stress-responsive MAPK pathways. Nat Cell Biol. 2008;10:1324–1332. - PubMed
-
- Cano CE, Hamidi T, Garcia MN, Grasso D, Loncle C, Garcia S, Calvo E, Lomberk G, Dusetti N, Bartholin L, et al. Genetic inactivation of Nupr1 acts as a dominant suppressor event in a two-hit model of pancreatic carcinogenesis. Gut. 2014;63:984–995. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

