Chemical and biological characterization of vaccine adjuvant QS-21 produced via plant cell culture
- PMID: 38361610
- PMCID: PMC10867646
- DOI: 10.1016/j.isci.2024.109006
Chemical and biological characterization of vaccine adjuvant QS-21 produced via plant cell culture
Abstract
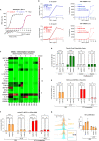
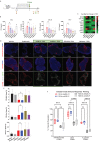
Many vaccines, including those using recombinant antigen subunits, rely on adjuvant(s) to enhance the efficacy of the host immune responses. Among the few adjuvants clinically approved, QS-21, a saponin-based immunomodulatory molecule isolated from the tree bark of Quillaja saponaria (QS) is used in complex formulations in approved effective vaccines. High demand of the QS raw material as well as manufacturing scalability limitation has been barriers here. We report for the first-time successful plant cell culture production of QS-21 having structural, chemical, and biologic, properties similar to the bark extracted product. These data ensure QS-21 and related saponins are broadly available and accessible to drug developers.
Keywords: Bioactive plant product; Phytochemistry; cell; medical biochemistry; natural product synthesis.
© 2024 The Author(s).
Conflict of interest statement
Some contributors declare potential conflict of interest. A.T.; X.L.; J.M.; H.H.; B.J.; M.W.; S.R.; B.K.; J.K.; G.L.; E.S.; J.H.; J.D.; J.C.; J.B.; C.K.; D.L.; and M.F. are actual or former employees or advisors for Agenus, Inc or SaponiQx, a subsidiary company of Agenus specialized in the development of saponin derived adjuvants. D.A.U.; T.L.; J.S.J.; and G.G. are actual or former employees of Phyton Biotech.
Figures





Similar articles
-
Synthesis and structure verification of the vaccine adjuvant QS-7-Api. Synthetic access to homogeneous Quillaja saponaria immunostimulants.J Am Chem Soc. 2008 May 7;130(18):5860-1. doi: 10.1021/ja801008m. Epub 2008 Apr 15. J Am Chem Soc. 2008. PMID: 18410100 Free PMC article.
-
Effect of a novel saponin adjuvant derived from Quillaja saponaria on the immune response to recombinant hepatitis B surface antigen.Mol Cells. 1997 Apr 30;7(2):178-86. Mol Cells. 1997. PMID: 9163729
-
QS-21 Adjuvant: Laboratory-Scale Purification Method and Formulation Into Liposomes.Methods Mol Biol. 2017;1494:73-86. doi: 10.1007/978-1-4939-6445-1_5. Methods Mol Biol. 2017. PMID: 27718186
-
Natural and synthetic saponin adjuvant QS-21 for vaccines against cancer.Expert Rev Vaccines. 2011 Apr;10(4):463-70. doi: 10.1586/erv.11.18. Expert Rev Vaccines. 2011. PMID: 21506644 Free PMC article. Review.
-
Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review.Phytomedicine. 2019 Jul;60:152905. doi: 10.1016/j.phymed.2019.152905. Epub 2019 Mar 30. Phytomedicine. 2019. PMID: 31182297 Free PMC article. Review.
Cited by
-
Cadmium and Copper Stress Responses in Soapbark Tree (Quillaja saponaria): Effects on Growth, Metal Accumulation, Saponin Concentration, and Gene Expression.Plants (Basel). 2025 Feb 26;14(5):709. doi: 10.3390/plants14050709. Plants (Basel). 2025. PMID: 40094634 Free PMC article.
-
A QS21 + CpG-Adjuvanted Trivalent HSV-2 Vaccine and Trivalent HSV-2 mRNA Vaccine Induce a Strong Immune Response, Protect Against HSV-2 Infection, and Cross-Protect Against HSV-1 Infection in Mice.Vaccines (Basel). 2025 May 6;13(5):497. doi: 10.3390/vaccines13050497. Vaccines (Basel). 2025. PMID: 40432109 Free PMC article.
-
Development of a New Vaccine Adjuvant System Based on the Combination of the Synthetic TLR4 Agonist FP20 and a Synthetic QS-21 Variant.J Med Chem. 2024 Dec 26;67(24):22254-22262. doi: 10.1021/acs.jmedchem.4c02392. Epub 2024 Dec 8. J Med Chem. 2024. PMID: 39645607 Free PMC article.
-
Plant Cell Culture-Derived Saponin Adjuvant Enhances Immune Response Against a Stabilized Human Metapneumovirus Pre-Fusion Vaccine Candidate.Vaccines (Basel). 2024 Dec 20;12(12):1435. doi: 10.3390/vaccines12121435. Vaccines (Basel). 2024. PMID: 39772095 Free PMC article.
-
Vaccines for Respiratory Viruses-COVID and Beyond.Vaccines (Basel). 2024 Aug 22;12(8):936. doi: 10.3390/vaccines12080936. Vaccines (Basel). 2024. PMID: 39204059 Free PMC article. Review.
References
-
- den Brok M.H., Büll C., Wassink M., de Graaf A.M., Wagenaars J.A., Minderman M., Thakur M., Amigorena S., Rijke E.O., Schrier C.C., Adema G.J. Saponin-based adjuvants induce cross-presentation in dendritic cells by intracellular lipid body formation. Nat. Commun. 2016;7 doi: 10.1038/ncomms13324. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

