Recognition of yeast β-glucan particles triggers immunometabolic signaling required for trained immunity
- PMID: 38361630
- PMCID: PMC10865028
- DOI: 10.1016/j.isci.2024.109030
Recognition of yeast β-glucan particles triggers immunometabolic signaling required for trained immunity
Abstract
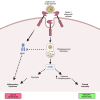
Fungal β-glucans are major drivers of trained immunity which increases long-term protection against secondary infections. Heterogeneity in β-glucan source, structure, and solubility alters interaction with the phagocytic receptor Dectin-1 and could impact strategies to improve trained immunity in humans. Using a panel of diverse β-glucans, we describe the ability of a specific yeast-derived whole-glucan particle (WGP) to reprogram metabolism and thereby drive trained immunity in human monocyte-derived macrophages in vitro and mice bone marrow in vivo. Presentation of pure, non-soluble, non-aggregated WGPs led to the formation of the Dectin-1 phagocytic synapse with subsequent lysosomal mTOR activation, metabolic reprogramming, and epigenetic rewiring. Intraperitoneal or oral administration of WGP drove bone marrow myelopoiesis and improved mature macrophage responses, pointing to therapeutic and food-based strategies to drive trained immunity. Thus, the investment of a cell in a trained response relies on specific recognition of β-glucans presented on intact microbial particles through stimulation of the Dectin-1 phagocytic response.
Keywords: Immunology; Molecular biology; Physiology.
© 2024 The Author(s).
Conflict of interest statement
M.L.E.L. is an employee of Kerry Group. F.J.S. is an applicant on an EU Priority Patent Application Patent Corporation Treaty application PI3885EPOO.
Figures






References
-
- Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E., Joosten L.A.B., van der Meer J.W.M., Mhlanga M.M., Mulder W.J.M., et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. - DOI - PMC - PubMed
-
- Quintin J., Saeed S., Martens J.H.A., Giamarellos-Bourboulis E.J., Ifrim D.C., Logie C., Jacobs L., Jansen T., Kullberg B.J., Wijmenga C., et al. Candida albicans infection affords protection against reinfection via functional reprogramming of monocytes. Cell Host Microbe. 2012;12:223–232. doi: 10.1016/j.chom.2012.06.006. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

