Specificities and redundancies in the NEL family of bacterial E3 ubiquitin ligases of Salmonella enterica serovar Typhimurium
- PMID: 38361917
- PMCID: PMC10867120
- DOI: 10.3389/fimmu.2024.1328707
Specificities and redundancies in the NEL family of bacterial E3 ubiquitin ligases of Salmonella enterica serovar Typhimurium
Abstract
Salmonella enterica serovar Typhimurium expresses two type III secretion systems, T3SS1 and T3SS2, which are encoded in Salmonella pathogenicity island 1 (SPI1) and SPI2, respectively. These are essential virulent factors that secrete more than 40 effectors that are translocated into host animal cells. This study focuses on three of these effectors, SlrP, SspH1, and SspH2, which are members of the NEL family of E3 ubiquitin ligases. We compared their expression, regulation, and translocation patterns, their role in cell invasion and intracellular proliferation, their ability to interact and ubiquitinate specific host partners, and their effect on cytokine secretion. We found that transcription of the three genes encoding these effectors depends on the virulence regulator PhoP. Although the three effectors have the potential to be secreted through T3SS1 and T3SS2, the secretion of SspH1 and SspH2 is largely restricted to T3SS2 due to their expression pattern. We detected a role for these effectors in proliferation inside fibroblasts that is masked by redundancy. The generation of chimeric proteins allowed us to demonstrate that the N-terminal part of these proteins, containing the leucine-rich repeat motifs, confers specificity towards ubiquitination targets. Furthermore, the polyubiquitination patterns generated were different for each effector, with Lys48 linkages being predominant for SspH1 and SspH2. Finally, our experiments support an anti-inflammatory role for SspH1 and SspH2.
Keywords: E3 ubiquitin ligase; Salmonella enterica; SlrP; SspH1; SspH2; type III secretion systems.
Copyright © 2024 Bullones-Bolaños, Martín-Muñoz, Vallejo-Grijalba, Bernal-Bayard and Ramos-Morales.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



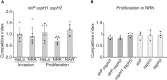




Similar articles
-
Salmonella typhimurium leucine-rich repeat proteins are targeted to the SPI1 and SPI2 type III secretion systems.Mol Microbiol. 1999 Nov;34(4):850-64. doi: 10.1046/j.1365-2958.1999.01651.x. Mol Microbiol. 1999. PMID: 10564523
-
SNRPD2 Is a Novel Substrate for the Ubiquitin Ligase Activity of the Salmonella Type III Secretion Effector SlrP.Biology (Basel). 2022 Oct 17;11(10):1517. doi: 10.3390/biology11101517. Biology (Basel). 2022. PMID: 36290420 Free PMC article.
-
Patterns of expression and translocation of the ubiquitin ligase SlrP in Salmonella enterica serovar Typhimurium.J Bacteriol. 2014 Nov;196(22):3912-22. doi: 10.1128/JB.02158-14. Epub 2014 Sep 2. J Bacteriol. 2014. PMID: 25182488 Free PMC article.
-
[Advances in Salmonella pathogenicity island 2 type III secretion system - A review].Wei Sheng Wu Xue Bao. 2016 Apr 4;56(4):561-9. Wei Sheng Wu Xue Bao. 2016. PMID: 29717847 Review. Chinese.
-
The NEL Family of Bacterial E3 Ubiquitin Ligases.Int J Mol Sci. 2022 Jul 13;23(14):7725. doi: 10.3390/ijms23147725. Int J Mol Sci. 2022. PMID: 35887072 Free PMC article. Review.
Cited by
-
Experimental Approaches to Visualize Effector Protein Translocation During Host-Pathogen Interactions.Bioessays. 2025 Apr;47(4):e202400188. doi: 10.1002/bies.202400188. Epub 2025 Mar 13. Bioessays. 2025. PMID: 40078034 Free PMC article. Review.
-
New insights into the putative role of leucine-rich repeat proteins of Leptospira interrogans and their participation in host cell invasion: an in silico analysis.Front Cell Infect Microbiol. 2024 Dec 13;14:1492352. doi: 10.3389/fcimb.2024.1492352. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39735260 Free PMC article. Review.
-
Salmonella Type III Secretion System Effectors.Int J Mol Sci. 2025 Mar 14;26(6):2611. doi: 10.3390/ijms26062611. Int J Mol Sci. 2025. PMID: 40141253 Free PMC article. Review.
-
The Salmonella Effector SspH2 Facilitates Spatially Selective Ubiquitination of NOD1 to Enhance Inflammatory Signaling.Biochemistry. 2024 Sep 17;63(18):2266-2279. doi: 10.1021/acs.biochem.4c00380. Epub 2024 Aug 27. Biochemistry. 2024. PMID: 39189508 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

