Mechanisms by which Factor H protects Trypanosoma cruzi from the alternative pathway of complement
- PMID: 38361922
- PMCID: PMC10867245
- DOI: 10.3389/fimmu.2024.1152000
Mechanisms by which Factor H protects Trypanosoma cruzi from the alternative pathway of complement
Abstract
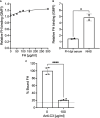
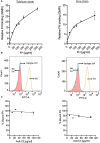
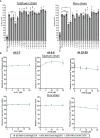
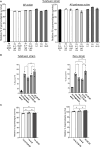
Chagas disease, a chronic disabling disease caused by the protozoan Trypanosoma cruzi, has no standardized treatment or preventative vaccine. The infective trypomastigote form of T. cruzi is highly resistant to killing by the complement immune system. Factor H (FH), a negative regulator of the alternative pathway (AP) of complement on cell surfaces and in blood, contains 20 short consensus repeat domains. The four N-terminal domains of FH inactivate the AP, while the other domains interact with C3b/d and glycan markers on cell surfaces. Various pathogens bind FH to inactivate the AP. T. cruzi uses its trans-sialidase enzyme to transfer host sialic acids to its own surface, which could be one of the approaches it uses to bind FH. Previous studies have shown that FH binds to complement-opsonized T. cruzi and parasite desialylation increases complement-mediated lysis of trypomastigotes. However, the molecular basis of FH binding to T. cruzi remain unknown. Only trypomastigotes, but not epimastigotes (non-infective, complement susceptible) bound FH directly, independent of C3 deposition, in a dose-dependent manner. Domain mapping experiments using 3-5 FH domain fragments showed that domains 5-8 competitively inhibited FH binding to the trypomastigotes by ~35% but did not decrease survival in complement. FH-Fc or mutant FH-Fc fusion proteins (3-11 contiguous FH domains fused to the IgG Fc) also did not kill trypomastigotes. FH-related protein-5, whose domains bear significant sequence identity to all known polyanion-binding FH domains (6-7, 10-14, 19-20), fully inhibited FH binding to trypomastigotes and reduced trypomastigote survival to < 24% in the presence of serum. In conclusion, we have elucidated the role of FH in complement resistance of trypomastigotes.
Keywords: Factor H; Factor H-related protein-5; Trypanosoma cruzi; alternative pathway; evasion strategy.
Copyright © 2024 Menon, Ramirez-Toloza, Wycoff, Ehinger, Shaughnessy, Ram and Ferreira.
Conflict of interest statement
Author KW was employed by the company Planet Biotechnology, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Trypanosoma cruzi Evades the Complement System as an Efficient Strategy to Survive in the Mammalian Host: The Specific Roles of Host/Parasite Molecules and Trypanosoma cruzi Calreticulin.Front Microbiol. 2017 Sep 1;8:1667. doi: 10.3389/fmicb.2017.01667. eCollection 2017. Front Microbiol. 2017. PMID: 28919885 Free PMC article. Review.
-
Mechanism of resistance to lysis by the alternative complement pathway in Trypanosoma cruzi trypomastigotes: effect of specific monoclonal antibody.J Immunol. 1986 Sep 1;137(5):1623-8. J Immunol. 1986. PMID: 2943798
-
Lytic rabbit IgG for tissue culture trypomastigotes of Trypanosoma cruzi alters the extent and form of complement deposition.Exp Parasitol. 1989 Feb;68(2):160-7. doi: 10.1016/0014-4894(89)90093-3. Exp Parasitol. 1989. PMID: 2647504
-
Role of sialic acid in the resistance of Trypanosoma cruzi trypomastigotes to complement.J Immunol. 1994 Oct 1;153(7):3141-7. J Immunol. 1994. PMID: 8089492
-
Is It Possible to Intervene in the Capacity of Trypanosoma cruzi to Elicit and Evade the Complement System?Front Immunol. 2021 Dec 16;12:789145. doi: 10.3389/fimmu.2021.789145. eCollection 2021. Front Immunol. 2021. PMID: 34975884 Free PMC article. Review.
Cited by
-
Complement therapeutic Factor H-IgG proteins as pre-exposure prophylaxes against Lyme borreliae infections.bioRxiv [Preprint]. 2024 Sep 26:2024.09.26.615144. doi: 10.1101/2024.09.26.615144. bioRxiv. 2024. PMID: 39386713 Free PMC article. Preprint.
References
-
- Available at: https://www.cdc.gov/parasites/chagas/epi.html.
-
- Available at: https://www.who.int/health-topics/chagas-disease#tab=tab_1.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

