Case report: Efficacy of immunotherapy as conversion therapy in dMMR/MSI-H colorectal cancer: a case series and review of the literature
- PMID: 38361927
- PMCID: PMC10867218
- DOI: 10.3389/fimmu.2024.1352262
Case report: Efficacy of immunotherapy as conversion therapy in dMMR/MSI-H colorectal cancer: a case series and review of the literature
Abstract
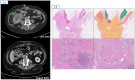
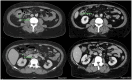
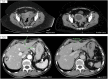
Immunotherapy has demonstrated a role in the therapeutic landscape of a small subset of patients with colorectal carcinoma (CRC) that harbor a microsatellite instability (MSI-H) status due to a deficient DNA mismatch repair (dMMR) system. The remarkable responses to immune checkpoint inhibitors (ICIs) are now being tested in the neoadjuvant setting in localized CRC, where the dMMR/MSI-H status can be found in up to 15% of patients, with remarkable results obtained in NICHE2 and 3 trials, among others. This case series aims to report our experience at a tertiary center and provide a comprehensive analysis of the possible questions and challenges to overcome if ICIs were established as standard of care in a neoadjuvant setting, as well as the potential role they may have as conversion therapy not only in locoregional advanced CRC but also in oligometastatic disease.
Keywords: colorectal cancer; conversion therapy; immunotherapy; microsatellite instability; neoadjuvant; oligometastatic.
Copyright © 2024 San-Román-Gil, Martínez-Delfrade, Albarrán-Fernández, Guerrero-Serrano, Pozas-Pérez, Chamorro-Pérez, Rosero-Rodríguez, Sotoca-Rubio, Barrill-Corpa, Alia-Navarro, González-Merino, García-de-Quevedo-Suero, López, Ruz-Caracuel, Perna-Monroy and Ferreiro-Monteagudo.
Conflict of interest statement
RF-M declares the following conflicts of interest—Advisory: Servier, Amgen, Pierre Fabre, Merck Sharp & Dohme (MSD); Speaker: Servier, Amgem, Merck, Pierre Fabre; Travels and other honoraria: Sanofi, Merck, Servier, Amgen. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

