Dietary docosahexaenoic acid supplementation inhibits acute pulmonary transcriptional and autoantibody responses to a single crystalline silica exposure in lupus-prone mice
- PMID: 38361937
- PMCID: PMC10867581
- DOI: 10.3389/fimmu.2024.1275265
Dietary docosahexaenoic acid supplementation inhibits acute pulmonary transcriptional and autoantibody responses to a single crystalline silica exposure in lupus-prone mice
Abstract
Introduction: Workplace exposure to respirable crystalline silica (cSiO2) has been epidemiologically linked to lupus. Consistent with this, repeated subchronic intranasal cSiO2 instillation in lupus-prone NZBWF1 mice induces inflammation-/autoimmune-related gene expression, ectopic lymphoid tissue (ELT), autoantibody (AAb) production in the lung within 5 to 13 wk followed systemic AAb increases and accelerated onset and progression of glomerulonephritis within 13 to 17 wk. Interestingly, dietary docosahexaenoic acid (DHA) supplementation suppresses these pathologic effects, but the underlying molecular mechanisms remain unclear.
Methods: This study aimed to test the hypothesis that dietary DHA supplementation impacts acute transcriptional and autoantibody responses in the lungs of female NZBWF1 mice 1 and 4 wk after a single high-dose cSiO2 challenge. Groups of mice were initially fed a control (Con) diet or a DHA-containing diet (10 g/kg). Cohorts of Con- and DHA-fed were subjected to a single intranasal instillation of 2.5 mg cSiO2 in a saline vehicle (Veh), while a Con-fed cohort was instilled with Veh only. At 1 and 4 wk post-instillation (PI), we compared cSiO2's effects on innate-/autoimmune-related gene expression and autoantibody (AAb) in lavage fluid/lungs of Con- and DHA-fed mice and related these findings to inflammatory cell profiles, histopathology, cell death, and cytokine/chemokine production.
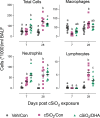
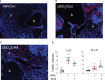
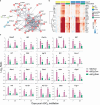
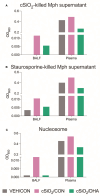
Results: DHA partially alleviated cSiO2-induced alterations in total immune cell and lymphocyte counts in lung lavage fluid. cSiO2-triggered dead cell accumulation and levels of inflammation-associated cytokines and IFN-stimulated chemokines were more pronounced in Con-fed mice than DHA-fed mice. Targeted multiplex transcriptome analysis revealed substantial upregulation of genes associated with autoimmune pathways in Con-fed mice in response to cSiO2 that were suppressed in DHA-fed mice. Pathway analysis indicated that DHA inhibited cSiO2 induction of proinflammatory and IFN-regulated gene networks, affecting key upstream regulators (e.g., TNFα, IL-1β, IFNAR, and IFNγ). Finally, cSiO2-triggered AAb responses were suppressed in DHA-fed mice.
Discussion: Taken together, DHA mitigated cSiO2-induced upregulation of pathways associated with proinflammatory and IFN-regulated gene responses within 1 wk and reduced AAb responses by 4 wk. These findings suggest that the acute short-term model employed here holds substantial promise for efficient elucidation of the molecular mechanisms through which omega-3 PUFAs exert protective effects against cSiO2-induced autoimmunity.
Keywords: autoantibody; autoimmune disease; crystalline silica; docosahexaenoic acid (DHA); inflammation; lung pathology; omega-3 fatty acid; systemic lupus erythematosus.
Copyright © 2024 Chauhan, Benninghoff, Favor, Wagner, Lewandowski, Rajasinghe, Li, Harkema and Pestka.
Conflict of interest statement
Author QL is currently employed by GeneCopoeia and author LR is currently employed by AstraZeneca. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures







Comment on
-
Rapid Induction of Pulmonary Inflammation, Autoimmune Gene Expression, and Ectopic Lymphoid Neogenesis Following Acute Silica Exposure in Lupus-Prone Mice.Front Immunol. 2021 Feb 23;12:635138. doi: 10.3389/fimmu.2021.635138. eCollection 2021. Front Immunol. 2021. PMID: 33732257 Free PMC article.
Similar articles
-
Omega-3 fatty acid intake suppresses induction of diverse autoantibody repertoire by crystalline silica in lupus-prone mice.Autoimmunity. 2020 Nov;53(7):415-433. doi: 10.1080/08916934.2020.1801651. Epub 2020 Sep 9. Autoimmunity. 2020. PMID: 32903098 Free PMC article.
-
Silica Induction of Diverse Inflammatory Proteome in Lungs of Lupus-Prone Mice Quelled by Dietary Docosahexaenoic Acid Supplementation.Front Immunol. 2022 Jan 21;12:781446. doi: 10.3389/fimmu.2021.781446. eCollection 2021. Front Immunol. 2022. PMID: 35126352 Free PMC article.
-
Dietary Docosahexaenoic Acid Prevents Silica-Induced Development of Pulmonary Ectopic Germinal Centers and Glomerulonephritis in the Lupus-Prone NZBWF1 Mouse.Front Immunol. 2018 Sep 12;9:2002. doi: 10.3389/fimmu.2018.02002. eCollection 2018. Front Immunol. 2018. PMID: 30258439 Free PMC article.
-
Lupus, Silica, and Dietary Omega-3 Fatty Acid Interventions.Toxicol Pathol. 2019 Dec;47(8):1004-1011. doi: 10.1177/0192623319878398. Epub 2019 Nov 14. Toxicol Pathol. 2019. PMID: 31725357 Free PMC article. Review.
-
Results from omic approaches in rat or mouse models exposed to inhaled crystalline silica: a systematic review.Part Fibre Toxicol. 2024 Mar 1;21(1):10. doi: 10.1186/s12989-024-00573-x. Part Fibre Toxicol. 2024. PMID: 38429797 Free PMC article.
Cited by
-
Supplementation of DHA enhances the cryopreservation of yak semen via alleviating oxidative stress and inhibiting apoptosis.Front Vet Sci. 2025 Feb 26;12:1532473. doi: 10.3389/fvets.2025.1532473. eCollection 2025. Front Vet Sci. 2025. PMID: 40078211 Free PMC article.
-
Impact of soluble epoxide hydrolase inhibition on silica-induced pulmonary fibrosis, ectopic lymphoid neogenesis, and autoantibody production in lupus-prone mice.Inhal Toxicol. 2024 Aug-Sep;36(7-8):442-460. doi: 10.1080/08958378.2024.2413373. Epub 2024 Oct 17. Inhal Toxicol. 2024. PMID: 39418113 Free PMC article.
References
-
- Conrad N, Misra S, Verbakel JY, Verbeke G, Molenberghs G, Taylor PN, et al. . Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: A population-based cohort study of 22 million individuals in the UK. Lancet (2023) 401(10391):1878–90. doi: 10.1016/S0140-6736(23)00457-9 - DOI - PubMed
-
- Bates MA, Brandenberger C, Langohr I, Kumagai K, Harkema JR, Holian A, et al. . Silica triggers inflammation and ectopic lymphoid neogenesis in the lungs in parallel with accelerated onset of systemic autoimmunity and glomerulonephritis in the lupus-prone NZBWF1 mouse. PloS One (2015) 10(5):1–25. doi: 10.1371/journal.pone.0125481 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

