Melatonin: the placental antioxidant and anti-inflammatory
- PMID: 38361952
- PMCID: PMC10867115
- DOI: 10.3389/fimmu.2024.1339304
Melatonin: the placental antioxidant and anti-inflammatory
Abstract
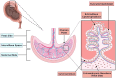
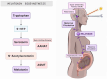
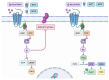
Melatonin (N-acetyl-5-methoxytryptamine) is an indolamine hormone with many physiological and biological roles. Melatonin is an antioxidant, anti-inflammatory, free radical scavenger, circadian rhythm regulator, and sleep hormone. However, its most popular role is the ability to regulate sleep through the circadian rhythm. Interestingly, recent studies have shown that melatonin is an important and essential hormone during pregnancy, specifically in the placenta. This is primarily due to the placenta's ability to synthesize its own melatonin rather than depending on the pineal gland. During pregnancy, melatonin acts as an antioxidant and anti-inflammatory, which is necessary to ensure a stable environment for both the mother and the fetus. It is an essential antioxidant in the placenta because it reduces oxidative stress by constantly scavenging for free radicals, i.e., maintain the placenta's integrity. In a healthy pregnancy, the maternal immune system is constantly altered to accommodate the needs of the growing fetus, and melatonin acts as a key anti-inflammatory by regulating immune homeostasis during early and late gestation. This literature review aims to identify and summarize melatonin's role as a powerful antioxidant and anti-inflammatory that reduces oxidative stress and inflammation to maintain a favorable homeostatic environment in the placenta throughout gestation.
Keywords: HCMV; NLRP3 inflammasome; anti-inflammatory; antioxidant; melatonin; oxidative stress; placenta; pregnancy.
Copyright © 2024 Joseph, Schuch, Hossack, Chakraborty and Johnson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

