Pharmacokinetics and pharmacodynamics across infusion rates of intravenously administered nipocalimab: results of a phase 1, placebo-controlled study
- PMID: 38362023
- PMCID: PMC10867144
- DOI: 10.3389/fnins.2024.1302714
Pharmacokinetics and pharmacodynamics across infusion rates of intravenously administered nipocalimab: results of a phase 1, placebo-controlled study
Abstract
Introduction: Nipocalimab is a high-affinity, fully human, aglycosylated, effectorless, immunoglobulin G (IgG) 1 monoclonal antibody that targets the neonatal Fc receptor (FcRn), decreases systemic IgG including autoantibodies, and is under development in several IgG autoantibody- and alloantibody-mediated diseases, including generalized myasthenia gravis, chronic inflammatory demyelinating polyneuropathy, maternal-fetal medicine, and multiple other therapeutic areas. An initial phase 1 study with single and multiple ascending doses of nipocalimab infused intravenously (IV) over 2 h demonstrated dose-dependent serum pharmacokinetics and IgG reductions, with an adverse event (AE) profile comparable to placebo.
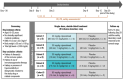
Methods: The current investigation evaluates the safety, tolerability, pharmacokinetics, and pharmacodynamics of single doses of nipocalimab across various IV infusion rates in a randomized, double-blind, placebo-controlled, sequential-dose study. Forty participants were randomized to receive nipocalimab 30 mg/kg over 60, 30, 15 or 7.5 min (0.5, 1, 2, or 4 mg/kg/min); nipocalimab 60 mg/kg over 15 min (4 mg/kg/min); or matching placebo.
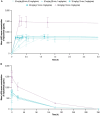
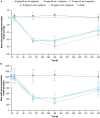
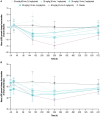
Results: At doses up to 60 mg/kg and infusion rates up to 4 mg/kg/min (maximum clinically feasible rate), single doses of nipocalimab were tolerable, with 12 (40%) participants experiencing AEs across nipocalimab cohorts compared with 1 (10%) participant in the placebo cohort. AEs deemed treatment related occurred in 6 (20%) participants receiving nipocalimab and 1 (10%) participant receiving placebo. None of the AEs were severe, and no participants discontinued treatment due to AEs. Nipocalimab provided consistent, dose-dependent serum pharmacokinetics and IgG reductions, regardless of infusion rate.
Discussion: This study supports the use of shortened durations of nipocalimab infusion for future studies.
Keywords: immunoglobulin G; intravenous infusions; monoclonal antibodies; neonatal Fc receptor; pharmacokinetics; phase 1 clinical trial.
Copyright © 2024 Leu, Vermeulen, Abbes, Arroyo, Denney and Ling.
Conflict of interest statement
JHL, AV, and LEL are employees of Janssen Research & Development, LLC and hold stock/stock options from Johnson & Johnson. CA and WSD were consultants for Momenta Pharmaceuticals, Inc. during this study and for Janssen during interpretation of the study. SA was an employee of Momenta Pharmaceuticals, Inc. during this study and a consultant for Janssen during interpretation of the study.
Figures
References
-
- Antozzi C., Guptill J., Bril V., Gamez J., Meuth S. G., Quan D., et al. . (2024). Safety and efficacy of nipocalimab in patients with generalized myasthenia gravis: results from the randomized phase 2 vivacity-MG study. Neurology 102:e207937. doi: 10.1212/WNL.0000000000207937, PMID: - DOI - PMC - PubMed
-
- Bril V., Druzdz A., Grosskreutz J., Habib A. A., Mantegazza R., Sacconi S., et al. . (2023). Safety and efficacy of rozanolixizumab in patients with generalised myasthenia gravis (MycarinG): a randomised, double-blind, placebo-controlled, adaptive phase 3 study. Lancet Neurol. 22, 383–394. doi: 10.1016/S1474-4422(23)00077-7, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

