Antineoplastic indole-containing compounds with potential VEGFR inhibitory properties
- PMID: 38362086
- PMCID: PMC10866129
- DOI: 10.1039/d3ra08962b
Antineoplastic indole-containing compounds with potential VEGFR inhibitory properties
Abstract
Cancer is one of the most significant health challenges worldwide. Various techniques, tools and therapeutics/materials have been developed in the last few decades for the treatment of cancer, together with great interest, funding and efforts from the scientific society. However, all the reported studies and efforts seem insufficient to combat the various types of cancer, especially the advanced ones. The overexpression of tyrosine kinases is associated with cancer proliferation and/or metastasis. VEGF, an important category of tyrosine kinases, and its receptors (VEGFR) are hyper-activated in different cancers. Accordingly, they are known as important factors in the angiogenesis of different tumors and are considered in the development of effective therapeutic approaches for controlling many types of cancer. In this case, targeted therapeutic approaches are preferable to the traditional non-selective approaches to minimize the side effects and drawbacks associated with treatment. Several indole-containing compounds have been identified as effective agents against VEGFR. Herein, we present a summary of the recent indolyl analogs reported within the last decade (2012-2023) with potential antineoplastic and VEGFR inhibitory properties. The most important drugs, natural products, synthesized potent compounds and promising hits/leads are highlighted. Indoles functionalized and conjugated with various heterocycles beside spiroindoles are also considered.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There is no conflict to declare.
Figures

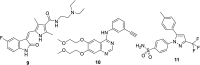
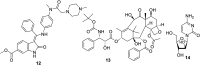
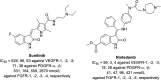
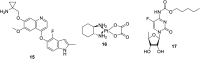
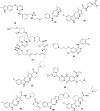
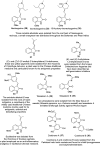
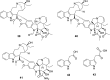



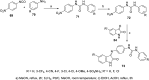
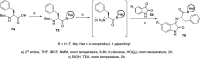
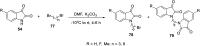
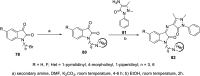
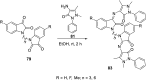
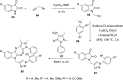
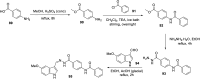
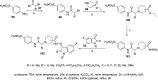
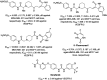




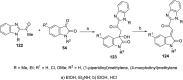
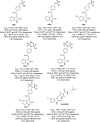
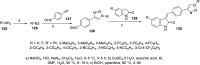

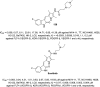

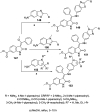


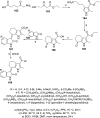

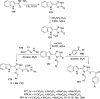

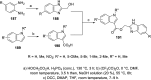
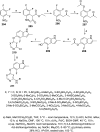
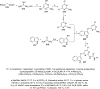
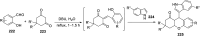
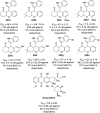





References
-
- Zha G.-F. Qin H.-L. Youssif B. G. M. Amjad M. W. Raja M. A. G. Abdelazeem A. H. Bukhari S. N. A. Discovery of potential anticancer multi-targeted ligustrazine based cyclohexanone and oxime analogs overcoming the cancer multidrug resistance. Eur. J. Med. Chem. 2017;135:34–48. doi: 10.1016/j.ejmech.2017.04.025. - DOI - PubMed
-
- Chhikara B. S. Parang K. Global cancer statistics 2022: the trends projection analysis. Chem. Biol. Lett. 2023;10:451.
Publication types
LinkOut - more resources
Full Text Sources

