The role of BAFF and APRIL in IgA nephropathy: pathogenic mechanisms and targeted therapies
- PMID: 38362118
- PMCID: PMC10867227
- DOI: 10.3389/fneph.2023.1346769
The role of BAFF and APRIL in IgA nephropathy: pathogenic mechanisms and targeted therapies
Abstract
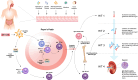
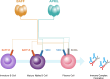
Immunoglobulin A nephropathy (IgAN), characterized by mesangial deposition of galactose-deficient-IgA1 (Gd-IgA1), is the most common biopsy-proven primary glomerulonephritis worldwide. Recently, an improved understanding of its underlying pathogenesis and the substantial risk of progression to kidney failure has emerged. The "four-hit hypothesis" of IgAN pathogenesis outlines a process that begins with elevated circulating levels of Gd-IgA1 that trigger autoantibody production. This results in the formation and deposition of immune complexes in the mesangium, leading to inflammation and kidney injury. Key mediators of the production of Gd-IgA1 and its corresponding autoantibodies are B-cell activating factor (BAFF), and A proliferation-inducing ligand (APRIL), each playing essential roles in the survival and maintenance of B cells and humoral immunity. Elevated serum levels of both BAFF and APRIL are observed in patients with IgAN and correlate with disease severity. This review explores the complex pathogenesis of IgAN, highlighting the pivotal roles of BAFF and APRIL in the interplay between mucosal hyper-responsiveness, B-cell activation, and the consequent overproduction of Gd-IgA1 and its autoantibodies that are key features in this disease. Finally, the potential therapeutic benefits of inhibiting BAFF and APRIL in IgAN, and a summary of recent clinical trial data, will be discussed.
Keywords: B-cell activating factor BAFF; IgA nephropathy; a proliferation-inducing ligand APRIL; atacicept; dual inhibition.
Copyright © 2024 Cheung, Barratt, Liew, Zhang, Tesar and Lafayette.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

