Protein dynamics of a light-driven Na+ pump rhodopsin probed using a tryptophan residue near the retinal chromophore
- PMID: 38362331
- PMCID: PMC10865881
- DOI: 10.2142/biophysico.bppb-v20.s016
Protein dynamics of a light-driven Na+ pump rhodopsin probed using a tryptophan residue near the retinal chromophore
Abstract
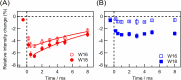
Direct observation of protein structural changes during ion transport in ion pumps provides valuable insights into the mechanism of ion transport. In this study, we examined structural changes in the light-driven sodium ion (Na+) pump rhodopsin KR2 on the sub-millisecond time scale, corresponding with the uptake and release of Na+. We compared the ion-pumping activities and transient absorption spectra of WT and the W215F mutant, in which the Trp215 residue located near the retinal chromophore on the cytoplasmic side was replaced with a Phe residue. Our findings indicated that atomic contacts between the bulky side chain of Trp215 and the C20 methyl group of the retinal chromophore promote relaxation of the retinal chromophore from the 13-cis to the all-trans form. Since Trp215 is conserved in other ion-pumping rhodopsins, the present results suggest that this residue commonly acts as a mechanical transducer. In addition, we measured time-resolved ultraviolet resonance Raman (UVRR) spectra to show that the environment around Trp215 becomes less hydrophobic at 1 ms after photoirradiation and recovers to the unphotolyzed state with a time constant of around 10 ms. These time scales correspond to Na+ uptake and release, suggesting evolution of a transient ion channel at the cytoplasmic side for Na+ uptake, consistent with the alternating-access model of ion pumps. The time-resolved UVRR technique has potential for application to other ion-pumping rhodopsins and could provide further insights into the mechanism of ion transport.
Keywords: alternating-access model; microbial rhodopsin; sodium ion pump; time-resolved resonance Raman spectroscopy.
2023 THE BIOPHYSICAL SOCIETY OF JAPAN.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures





References
-
- Drew, D., Boudker, O.. Shared molecular mechanisms of membrane transporters. Annu. Rev. Biochem. 85, 543–572 (2016). https://doi.org/10.1146/annurev-biochem-060815-014520 - PubMed
-
- Kandori, H. Ion-pumping microbial rhodopsins. Front. Mol. Biosci. 2 (2015). https://doi.org/10.3389/fmolb.2015.00052 - PMC - PubMed
-
- Rozenberg, A., Inoue, K., Kandori, H., Béjà, O.. Microbial rhodopsins: The last two decades. Annu. Rev. Microbiol. 75, 427–447 (2021). https://doi.org/10.1146/annurev-micro-031721-020452 - PubMed
-
- Ernst, O. P., Lodowski, D. T., Elstner, M., Hegemann, P., Brown, L. S., Kandori, H.. Microbial and animal rhodopsins: Structures, functions, and molecular mechanisms. Chem. Rev. 114, 126–163 (2014). https://doi.org/10.1021/cr4003769 - PMC - PubMed
-
- Mizutani, Y. Concerted motions and molecular function: What physical chemistry we can learn from light-driven ion-pumping rhodopsins. J. Phys. Chem. B 125, 11812–11819 (2021). https://doi.org/10.1021/acs.jpcb.1c06698 - PubMed

