Endothelial Overexpression of TGF-β-Induced Protein Impairs Venous Thrombus Resolution: Possible Role in CTEPH
- PMID: 38362348
- PMCID: PMC10864968
- DOI: 10.1016/j.jacbts.2023.08.005
Endothelial Overexpression of TGF-β-Induced Protein Impairs Venous Thrombus Resolution: Possible Role in CTEPH
Abstract
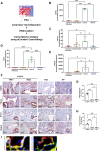
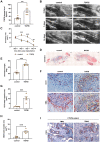
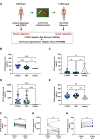
Endothelial cells play a critical role during venous thrombus remodeling, and unresolved, fibrotic thrombi with irregular vessels obstruct the pulmonary artery in patients with chronic thromboembolic pulmonary hypertension (CTEPH). This study sought to identify endothelial mediators of impaired venous thrombus resolution and to determine their role in the pathogenesis of the vascular obstructions in patients with CTEPH. Endothelial cells outgrown from pulmonary endarterectomy specimens (PEA) were processed for mRNA profiling, and nCounter gene expression and immunohistochemistry analysis of PEA tissue microarrays and immunoassays of plasma were used to validate the expression in CTEPH. Lentiviral overexpression in human pulmonary artery endothelial cells (HPAECs) and exogenous administration of the recombinant protein into C57BL/6J mice after inferior Vena cava ligation were employed to assess their role for venous thrombus resolution. RT2 PCR profiler analysis demonstrated the significant overexpression of factors downstream of transforming growth factor beta (TGFβ), that is TGFβ-Induced Protein (TGFBI or BIGH3) and transgelin (TAGLN), or involved in TGFβ signaling, that is follistatin-like 3 (FSTL3) and stanniocalcin-2 (STC2). Gene expression and immunohistochemistry analysis of tissue microarrays localized potential disease candidates to vessel-rich regions. Lentiviral overexpression of TGFBI in HPAECs increased fibrotic remodeling of human blood clots in vitro, and exogenous administration of recombinant TGFBI in mice delayed venous thrombus resolution. Significantly elevated plasma TGFBI levels were observed in patients with CTEPH and decreased after PEA. Our findings suggest that overexpression of TGFBI in endothelial promotes venous thrombus non-resolution and fibrosis and is causally involved in the pathophysiology of CTEPH.
Keywords: chronic thromboembolic pulmonary hypertension; endothelium; fibrosis; thrombosis; transforming growth factor beta-induced.
© 2024 The Authors.
Conflict of interest statement
This study was supported by the Bundesministerium für Bildung und Forschung (BMBF 01E01003; Virchow fellowship and TRP-X15 to Dr Bochenek), the Deutsches Zentrum für Herz-Kreislauf-Forschung (cooperation with DZHK Shared-Expertise [SE]-105 and SE-006 to Drs Saar and Bochenek) and the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 1531, A9 to Dr Saar; project number 456687919). Drs Münzel, Schober, Hübner, and Schäfer are principal investigators of the Deutsches Zentrum für Herz-Kreislauf-Forschung. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures



References
-
- Delcroix M., Torbicki A., Gopalan D., et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J. 2021;57 - PubMed
-
- Humbert M., Kovacs G., Hoeper M.M., et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43:3618–3731. - PubMed
-
- Bochenek M.L., Rosinus N.S., Lankeit M., et al. From thrombosis to fibrosis in chronic thromboembolic pulmonary hypertension. Thromb Haemost. 2017;117:769–783. - PubMed
-
- Hobohm L., Kolmel S., Niemann C., et al. Role of angiopoietin-2 in venous thrombus resolution and chronic thromboembolic disease. Eur Respir J. 2021;58 - PubMed
-
- Bochenek M.L., Schäfer K. Role of endothelial cells in acute and chronic thrombosis. Hamostaseologie. 2019;39:128–139. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

