Manganese-catalyzed base-free addition of saturated nitriles to unsaturated nitriles by template catalysis
- PMID: 38362414
- PMCID: PMC10866344
- DOI: 10.1039/d3sc04935c
Manganese-catalyzed base-free addition of saturated nitriles to unsaturated nitriles by template catalysis
Abstract
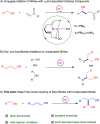
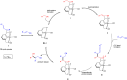
The coupling of mononitriles into dinitriles is a desirable strategy, given the prevalence of nitrile compounds and the synthetic and industrial utility of dinitriles. Herein, we present an atom-economical approach for the heteroaddition of saturated nitriles to α,β- and β,γ-unsaturated mononitriles to generate glutaronitrile derivatives using a catalyst based on earth-abundant manganese. A broad range of such saturated and unsaturated nitriles were found to undergo facile heteroaddition with excellent functional group tolerance, in a reaction that proceeds under mild and base-free conditions using low catalyst loading. Mechanistic studies showed that this unique transformation takes place through a template-type pathway involving an enamido complex intermediate, which is generated by addition of a saturated nitrile to the catalyst, and acts as a nucleophile for Michael addition to unsaturated nitriles. This work represents a new application of template catalysis for C-C bond formation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Template Catalysis by Metal-Ligand Cooperation. C-C Bond Formation via Conjugate Addition of Non-activated Nitriles under Mild, Base-free Conditions Catalyzed by a Manganese Pincer Complex.J Am Chem Soc. 2016 Jun 8;138(22):6985-97. doi: 10.1021/jacs.5b13208. Epub 2016 May 24. J Am Chem Soc. 2016. PMID: 27164437
-
Template catalysis by manganese pincer complexes: oxa- and aza-Michael additions to unsaturated nitriles.Chem Sci. 2019 Aug 7;10(39):8990-8994. doi: 10.1039/c9sc03269j. eCollection 2019 Oct 21. Chem Sci. 2019. PMID: 31762979 Free PMC article.
-
Manganese-Ketenimine Intermediates as Active Catalysts in the Michael Addition of Unactivated Nitriles to α,β-Unsaturated Ketones.Angew Chem Int Ed Engl. 2025 Apr 25;64(18):e202423275. doi: 10.1002/anie.202423275. Epub 2025 Mar 25. Angew Chem Int Ed Engl. 2025. PMID: 39968599
-
Manganese Alkyl Carbonyl Complexes: From Iconic Stoichiometric Textbook Reactions to Catalytic Applications.Acc Chem Res. 2022 Sep 20;55(18):2740-2751. doi: 10.1021/acs.accounts.2c00470. Epub 2022 Sep 8. Acc Chem Res. 2022. PMID: 36074912 Free PMC article. Review.
-
A review on the assembly of multi-substituted pyridines via Co-catalyzed [2 + 2 + 2] cycloaddition with nitriles.Org Biomol Chem. 2022 Sep 28;20(37):7391-7404. doi: 10.1039/d2ob01344d. Org Biomol Chem. 2022. PMID: 36098263 Review.
Cited by
-
Broadly Applicable Copper(I)-Catalyzed Alkyne Semihydrogenation and Hydrogenation of α,β-Unsaturated Amides Enabled by Bifunctional Iminopyridine Ligands.J Am Chem Soc. 2025 Apr 30;147(17):14481-14490. doi: 10.1021/jacs.5c01339. Epub 2025 Apr 16. J Am Chem Soc. 2025. PMID: 40239054 Free PMC article.
References
-
- Ló pez R. Palomo C. Angew. Chem., Int. Ed. 2015;54:13170–13184. doi: 10.1002/anie.201502493. - DOI - PubMed
- Fleming F. F. Yao L. Ravikumar P. C. Funk L. Shook B. C. J. Med. Chem. 2010;53:7902–7917. doi: 10.1021/jm100762r. - DOI - PMC - PubMed
- Fleming F. F. Nat. Prod. Rep. 1999;16:597–606. doi: 10.1039/A804370A. - DOI
-
- Ullmann's encyclopedia of industrial chemistry, ed B. Elvers and F. Ullmann, Wiley-VCH, Weinheim, 2011
- Arpe H.-J., Industrial Organic Chemistry, Wiley-VCH, Weinheim, 5th edn, 2010, ch. 10
-
-
Reviews:
- Scotti C. Barlow J. W. Nat. Prod. Commun. 2022;17:1–24. doi: 10.1177/1934578X221099973. - DOI
- Rakshit A. Dhara H. N. Sahoo A. K. Patel B. K. Chem.–Asian J. 2022;17:e202200792. doi: 10.1002/asia.202200792. - DOI - PubMed
- Xia Y. Jiang zH. Wu W. Eur. J. Org Chem. 2021;2021:6658–6669. doi: 10.1002/ejoc.202101196. - DOI
-
-
- Long J. Yu R. Gao J. Fang X. Angew. Chem., Int. Ed. 2020;59:6785–6789. doi: 10.1002/anie.202000704. - DOI - PubMed
- Sun F. Gao J. Fang X. Chem. Commun. 2020;56:6858–6861. doi: 10.1039/D0CC02938F. - DOI - PubMed
- Chen J. Wang P.-Z. Lu B. Liang D. Yu X.-Y. Xiao W.-J. Chen J.-R. Org. Lett. 2019;21:9763–9768. doi: 10.1021/acs.orglett.9b03970. - DOI - PubMed
- Wang Y. Liu X. Deng L. J. Am. Chem. Soc. 2006;128:3928–3930. doi: 10.1021/ja060312n. - DOI - PubMed
-
- Long J. Xia S. Wang T. Cheng G.-J. Fang X. ACS Catal. 2021;11:13880–13890. doi: 10.1021/acscatal.1c03729. - DOI
- Qi L. Li R. Yao X. Zhen Q. Ye P. Shao Y. Chen J. J. Org. Chem. 2020;85:1097–1108. doi: 10.1021/acs.joc.9b02999. - DOI - PubMed
- Wang T. Wang Y.-N. Wang R. Zhang B.-C. Yang C. Li Y.-L. Wang X.-S. Nat. Commun. 2019;10:5373. doi: 10.1038/s41467-019-13369-x. - DOI - PMC - PubMed
- Laval S. Dayoub W. Pehlivan L. Métay E. Favre-Reguillon A. Delbrayelle D. Mignani G. Lemaire M. Tetrahedron. 2014;70:975–983. doi: 10.1016/j.tet.2013.11.101. - DOI
- Hartmann R. W. Batzl C. J. Med. Chem. 1986;29:1362–1369. doi: 10.1021/jm00158a007. - DOI - PubMed
LinkOut - more resources
Full Text Sources

