All-catecholate-stabilized black titanium-oxo clusters for efficient photothermal conversion
- PMID: 38362423
- PMCID: PMC10866351
- DOI: 10.1039/d3sc05617a
All-catecholate-stabilized black titanium-oxo clusters for efficient photothermal conversion
Abstract
The controlled synthesis of titanium-oxo clusters (TOCs) completely stabilized by organic dye ligands with high stability and superior light absorption remains a significant challenge. In this study, we report the syntheses of three atomically precise catechol (Cat)-functionalized TOCs, [Ti2(Cat)2(OEgO)2(OEgOH)2] (Ti2), [Ti8O5(Cat)9(iPrO)4(iPrOH)2] (Ti8), and [Ti16O8(OH)8(Cat)20]·H2O·PhMe (Ti16), using a solvent-induced strategy (HOEgOH = ethylene glycol; iPrOH = isopropanol; PhMe = toluene). Interestingly, the TiO core of Ti16 is almost entirely enveloped by catechol ligands, making it the first all-catechol-protected high-nuclearity TOC. In contrast, Ti2 and Ti8 have four weakly coordinated ethylene glycol ligands and six weakly coordinated iPrOH ligands, respectively, in addition to the catechol ligands. Ti16 is visually evident in its distinctively black appearance, which belongs to black TOCs (B-TOCs) and exhibits an ultralow optical band gap. Furthermore, Ti16 displays exceptional stability in various media/environments, including exposure to air, solvents, and both acidic and alkaline aqueous solutions due to its comprehensive protection by catechol ligands and rich intra-cluster supramolecular interactions. Ti16 has superior photoelectric response qualities and photothermal conversion capabilities compared to Ti2 and Ti8 due to its ultralow optical band gap and remarkable stability. This discovery not only represents a huge step forward in the creation of all-catecholate-protected B-TOCs with ultralow optical band gaps and outstanding stability, but it also gives key valuable mechanistic insights into their photothermal/electric applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

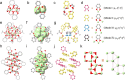




References
-
- Zhao C. Zhang Z. Han F. Xia D. Xiao C. Fang J. Zhang Y. Wu B. You S. Wu Y. Li W. An Organic–Inorganic Hybrid Electrolyte as a Cathode Interlayer for Efficient Organic Solar Cells. Angew. Chem., Int. Ed. 2021;133:8607–8612. - PubMed
-
- Xiao G.-B. Mu X. Zhou S. Zhu L. Peng Y. Liang Q. Zou X. Zhang J. Zhang L. Cao J. Directional Transformation of Heterometallic Oxo Clusters: A New Approach to Prepare Wide-Bandgap Cathode Interlayers for Perovskite Solar Cells. Angew. Chem., Int. Ed. 2023;62:e202218478. doi: 10.1002/anie.202218478. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

