Tolerating CD47
- PMID: 38362603
- PMCID: PMC10870051
- DOI: 10.1002/ctm2.1584
Tolerating CD47
Abstract
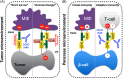
Cluster of differentiation 47 (CD47) occupies the outer membrane of human cells, where it binds to soluble and cell surface receptors on the same and other cells, sculpting their topography and resulting in a pleiotropic receptor-multiligand interaction network. It is a focus of drug development to temper and accentuate CD47-driven immune cell liaisons, although consideration of on-target CD47 effects remain neglected. And yet, a late clinical trial of a CD47-blocking antibody was discontinued, existent trials were restrained, and development of CD47-targeting agents halted by some pharmaceutical companies. At this point, if CD47 can be exploited for clinical advantage remains to be determined. Herein an airing is made of the seemingly conflicting actions of CD47 that reflect its position as a junction connecting receptors and signalling pathways that impact numerous human cell types. Prospects of CD47 boosting and blocking are considered along with potential therapeutic implications for autoimmune diseases and cancer.
Keywords: CD47; SIRPα; TSP1; autoimmunity; cancer; checkpoint inhibitor; immunotherapy.
© 2024 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
J.S.I. is a consultant to San Rocco Therapeutics, Tampa, FL. E.M. declares no conflicts of interest regarding the work.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
