Differential 5'-tRNA Fragment Expression in Circulating Preeclampsia Syncytiotrophoblast Vesicles Drives Macrophage Inflammation
- PMID: 38362745
- PMCID: PMC10956686
- DOI: 10.1161/HYPERTENSIONAHA.123.22292
Differential 5'-tRNA Fragment Expression in Circulating Preeclampsia Syncytiotrophoblast Vesicles Drives Macrophage Inflammation
Abstract
Background: The relationship between placental pathology and the maternal syndrome of preeclampsia is incompletely characterized. Mismatch between placental nutrient supply and fetal demands induces stress in the syncytiotrophoblast, the layer of placenta in direct contact with maternal blood. Such stress alters the content and increases the release of syncytiotrophoblast extracellular vesicles (STB-EVs) into the maternal circulation. We have previously shown 5'-tRNA fragments (5'-tRFs) constitute the majority of small RNA in STB-EVs in healthy pregnancy. 5'-tRFs are produced in response to stress. We hypothesized STB-EV 5'-tRF release might change in preeclampsia.
Methods: We perfused placentas from 8 women with early-onset preeclampsia and 6 controls, comparing small RNA expression in STB-EVs. We used membrane-affinity columns to isolate maternal plasma vesicles and investigate placental 5'-tRFs in vivo. We quantified 5'-tRFs from circulating STB-EVs using a placental alkaline phosphatase immunoassay. 5'-tRFs and scrambled RNA controls were added to monocyte, macrophage and endothelial cells in culture to investigate transcriptional responses.
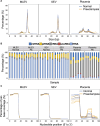
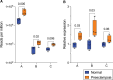
Results: 5'-tRFs constitute the majority of small RNA in STB-EVs from both preeclampsia and normal pregnancies. More than 900 small RNA fragments are differentially expressed in preeclampsia STB-EVs. Preeclampsia-dysregulated 5'-tRFs are detectable in maternal plasma, where we identified a placentally derived load. 5'-tRF-Glu-CTC, the most abundant preeclampsia-upregulated 5'-tRF in perfusion STB-EVs, is also increased in preeclampsia STB-EVs from maternal plasma. 5'-tRF-Glu-CTC induced inflammation in macrophages but not monocytes. The conditioned media from 5'-tRF-Glu-CTC-activated macrophages reduced eNOS (endothelial NO synthase) expression in endothelial cells.
Conclusions: Increased release of syncytiotrophoblast-derived vesicle-bound 5'-tRF-Glu-CTC contributes to preeclampsia pathophysiology.
Keywords: extracellular vesicles; macrophages; preeclampsia; transfer RNA.
Conflict of interest statement
Y.M.D. Lo holds equity in DRA, Insighta, Grail/Illumina and Take2. P. Jiang holds equity in Illumina. P. Jiang is a consultant to Take2. P. Jiang is a Director of Take2, Insighta, DRA and KingMed Future. Y.M.D. Lo , P. Jiang , and L. Ji receive royalties from Illumina, LabCorp, Grail, DRA, Xcelom and Take2.
Figures



References
-
- Magee LA, Nicolaides KH, von Dadelszen P. Preeclampsia. N Engl J Med. 2022;386:1817–1832. doi: 10.1056/NEJMra2109523 - PubMed
-
- Redman CWG, Staff AC, Roberts JM. Syncytiotrophoblast stress in preeclampsia: the convergence point for multiple pathways. Am J Obstet Gynecol. 2022;226:S907–S927. doi: 10.1016/j.ajog.2020.09.047 - PubMed
-
- Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, et al. . Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

