Profiling amyloid-β peptides as biomarkers for cerebral amyloid angiopathy
- PMID: 38362804
- PMCID: PMC11260253
- DOI: 10.1111/jnc.16074
Profiling amyloid-β peptides as biomarkers for cerebral amyloid angiopathy
Abstract
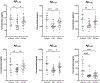
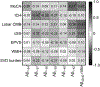
Brain amyloid-β (Aβ) deposits are key pathological hallmarks of both cerebral amyloid angiopathy (CAA) and Alzheimer's disease (AD). Microvascular deposits in CAA mainly consist of the Aβ40 peptide, whereas Aβ42 is the predominant variant in parenchymal plaques in AD. The relevance in pathogenesis and diagnostic accuracy of various other Aβ isoforms in CAA remain understudied. We aimed to investigate the biomarker potential of various Aβ isoforms in cerebrospinal fluid (CSF) to differentiate CAA from AD pathology. We included 25 patients with probable CAA, 50 subjects with a CSF profile indicative of AD pathology (AD-like), and 23 age- and sex-matched controls. CSF levels of Aβ1-34, Aβ1-37, Aβ1-38, Aβ1-39, Aβ1-40, and Aβ1-42 were quantified by liquid chromatography mass spectrometry. Lower CSF levels of all six Aβ peptides were observed in CAA patients compared with controls (p = 0.0005-0.03). Except for Aβ1-42 (p = 1.0), all peptides were decreased in CAA compared with AD-like subjects (p = 0.007-0.03). Besides Aβ1-42, none of the Aβ peptides were decreased in AD-like subjects compared with controls. All Aβ peptides combined differentiated CAA from AD-like subjects better (area under the curve [AUC] 0.84) than individual peptide levels (AUC 0.51-0.75). Without Aβ1-42 in the model (since decreased Aβ1-42 served as AD-like selection criterion), the AUC was 0.78 for distinguishing CAA from AD-like subjects. CAA patients and AD-like subjects showed distinct disease-specific CSF Aβ profiles. Peptides shorter than Aβ1-42 were decreased in CAA patients, but not AD-like subjects, which could suggest different pathological mechanisms between vascular and parenchymal Aβ accumulation. This study supports the potential use of this panel of CSF Aβ peptides to indicate presence of CAA pathology with high accuracy.
Keywords: Alzheimer's disease; amyloid‐β; biomarkers; cerebral amyloid angiopathy; cerebrospinal fluid; mass spectrometry.
© 2024 The Authors. Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
CONFLICTS OF INTEREST STATEMENT
HZ has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen, and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). ES is employee of ADx NeuroSciences. KB has served as a consultant, at advisory boards, or at data monitoring committees for BioArctic, Biogen, Julius Clinical, Lilly, Novartis, Ono Pharma, Prothena, Roche Diagnostics, and Siemens Healthineers, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. The other authors declare that they have no competing interests.
Figures
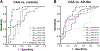
References
-
- DeSimone CV, Graff-Radford J, El-Harasis MA, Rabinstein AA, Asirvatham SJ, Holmes DR Jr. (2017) Cerebral amyloid angiopathy: Diagnosis, clinical implications, and management strategies in atrial fibrillation. J Am Coll Cardiol 70, 1173–1182. - PubMed
-
- Charidimou A, Boulouis G, Frosch MP, Baron JC, Pasi M, Albucher JF, Banerjee G, Barbato C, Bonneville F, Brandner S, Calviere L, Caparros F, Casolla B, Cordonnier C, Delisle MB, Deramecourt V, Dichgans M, Gokcal E, Herms J, Hernandez-Guillamon M, Jäger HR, Jaunmuktane Z, Linn J, Martinez-Ramirez S, Martínez-Sáez E, Mawrin C, Montaner J, Moulin S, Olivot JM, Piazza F, Puy L, Raposo N, Rodrigues MA, Roeber S, Romero JR, Samarasekera N, Schneider JA, Schreiber S, Schreiber F, Schwall C, Smith C, Szalardy L, Varlet P, Viguier A, Wardlaw JM, Warren A, Wollenweber FA, Zedde M, van Buchem MA, Gurol ME, Viswanathan A, Al-Shahi Salman R, Smith EE, Werring DJ, Greenberg SM (2022) The Boston criteria version 2.0 for cerebral amyloid angiopathy: A multicentre, retrospective, MRI-neuropathology diagnostic accuracy study. Lancet Neurol 21, 714–725. - PMC - PubMed
-
- Charidimou A, Boulouis G (2022) Clinical diagnosis of probable cerebral amyloid angiopathy: Diagnostic accuracy meta-analysis of the Boston criteria. Stroke 53, 3679–3687. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

