Resisting death by metal: metabolism and Cu/Zn homeostasis in bacteria
- PMID: 38362914
- PMCID: PMC10903455
- DOI: 10.1042/ETLS20230115
Resisting death by metal: metabolism and Cu/Zn homeostasis in bacteria
Abstract
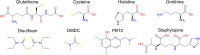
Metal ions such as zinc and copper play important roles in host-microbe interactions and their availability can drastically affect the survival of pathogenic bacteria in a host niche. Mechanisms of metal homeostasis protect bacteria from starvation, or intoxication, defined as when metals are limiting, or in excess, respectively. In this mini-review, we summarise current knowledge on the mechanisms of resistance to metal stress in bacteria, focussing specifically on the homeostasis of cellular copper and zinc. This includes a summary of the factors that subvert metal stress in bacteria, which are independent of metal efflux systems, and commentary on the role of small molecules and metabolic systems as important mediators of metal resistance.
Keywords: copper; homeostasis; metabolism; metal; small molecules; zinc.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

Similar articles
-
Recent developments in copper and zinc homeostasis in bacterial pathogens.Curr Opin Chem Biol. 2014 Apr;19:59-66. doi: 10.1016/j.cbpa.2013.12.021. Epub 2014 Jan 22. Curr Opin Chem Biol. 2014. PMID: 24463765 Free PMC article. Review.
-
The Copper Resistome of Group B Streptococcus Reveals Insight into the Genetic Basis of Cellular Survival during Metal Ion Stress.J Bacteriol. 2022 May 17;204(5):e0006822. doi: 10.1128/jb.00068-22. Epub 2022 Apr 11. J Bacteriol. 2022. PMID: 35404113 Free PMC article.
-
Diisonitrile Lipopeptides Mediate Resistance to Copper Starvation in Pathogenic Mycobacteria.mBio. 2022 Oct 26;13(5):e0251322. doi: 10.1128/mbio.02513-22. Epub 2022 Oct 5. mBio. 2022. PMID: 36197089 Free PMC article.
-
Regulatory cross-talk supports resistance to Zn intoxication in Streptococcus.PLoS Pathog. 2022 Jul 21;18(7):e1010607. doi: 10.1371/journal.ppat.1010607. eCollection 2022 Jul. PLoS Pathog. 2022. PMID: 35862444 Free PMC article.
-
Role of divalent metals in infectious disease susceptibility and outcome.Clin Microbiol Infect. 2018 Jan;24(1):16-23. doi: 10.1016/j.cmi.2017.01.018. Epub 2017 Jan 29. Clin Microbiol Infect. 2018. PMID: 28143784 Review.
Cited by
-
Microbial metal physiology: ions to ecosystems.Nat Rev Microbiol. 2025 Jul 25. doi: 10.1038/s41579-025-01213-7. Online ahead of print. Nat Rev Microbiol. 2025. PMID: 40715508 Review.
-
Comparative genomic analysis of metal-tolerant bacteria reveals significant differences in metal adaptation strategies.Microbiol Spectr. 2025 Jun 3;13(6):e0168024. doi: 10.1128/spectrum.01680-24. Epub 2025 Apr 24. Microbiol Spectr. 2025. PMID: 40272196 Free PMC article.
-
Advances in pH-responsive drug delivery systems for periodontitis treatment.Drug Deliv. 2025 Dec;32(1):2522109. doi: 10.1080/10717544.2025.2522109. Epub 2025 Jun 27. Drug Deliv. 2025. PMID: 40574628 Free PMC article. Review.
-
The Role of Ferroptosis and Cuproptosis in Tuberculosis Pathogenesis: Implications for Therapeutic Strategies.Curr Issues Mol Biol. 2025 Feb 5;47(2):99. doi: 10.3390/cimb47020099. Curr Issues Mol Biol. 2025. PMID: 39996820 Free PMC article. Review.
-
Bacteria Under Metal Stress-Molecular Mechanisms of Metal Tolerance.Int J Mol Sci. 2025 Jun 14;26(12):5716. doi: 10.3390/ijms26125716. Int J Mol Sci. 2025. PMID: 40565180 Free PMC article. Review.
References
-
- Irving, H. and Williams, R.J.P. (1953) The stability of transition-metal complexes. J. Chem. Soc. 0, 3192–3210 10.1039/jr9530003192 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
