Immune cells and inflammatory mediators cause endothelial dysfunction in a vascular microphysiological system
- PMID: 38363157
- PMCID: PMC11022267
- DOI: 10.1039/d3lc00824j
Immune cells and inflammatory mediators cause endothelial dysfunction in a vascular microphysiological system
Abstract
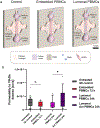
Functional assessment of endothelium serves as an important indicator of vascular health and is compromised in vascular disorders including hypertension, atherosclerosis, and preeclampsia. Endothelial dysfunction in these cases is linked to dysregulation of the immune system involving both changes to immune cells and increased secretion of inflammatory cytokines. Herein, we utilize a well-established microfluidic device to generate a 3-dimensional vascular microphysiological system (MPS) consisting of a tubular blood vessel lined with human umbilical vein endothelial cells (HUVECs) to evaluate endothelial function measured via endothelial permeability and Ca2+ signaling. We evaluated the effect of a mixture of factors associated with inflammation and cardiovascular disease (TNFα, VEGF-A, IL-6 at 10 ng ml-1 each) on vascular MPS and inferred that inflammatory mediators contribute to endothelial dysfunction by disrupting the endothelial barrier over a 48 hour treatment and by diminishing coordinated Ca2+ activity over a 1 hour treatment. We also evaluated the effect of peripheral blood mononuclear cells (PBMCs) on endothelial permeability and Ca2+ signaling in the HUVEC MPS. HUVECs were co-cultured with PBMCs either directly wherein PBMCs passed through the lumen or indirectly with PBMCs embedded in the supporting collagen hydrogel. We revealed that phytohemagglutinin (PHA)-M activated PBMCs cause endothelial dysfunction in MPS both through increased permeability and decreased coordinated Ca2+ activity compared to non-activated PBMCs. Our MPS has potential applications in modeling cardiovascular disorders and screening for potential treatments using measures of endothelial function.
Conflict of interest statement
CONFLICTS OF INTEREST
D. J. B. holds equity in BellBrook Labs LLC, Tasso Inc. Stacks to the Future LLC, Lynx Biosciences LLC, Onexio Biosystems LLC, Flambeau Diagnostics LLC, and Salus Discovery LLC. The remaining authors declare no competing financial interests.
Figures


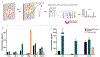



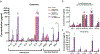
References
-
- Xu S, Ilyas I, Little PJ, Li H, Kamato D, Zheng X, Luo S, Li Z, Liu P, Han J, Harding IC, Ebong EE, Cameron SJ, Stewart AG and Weng J, Pharmacol Rev, 2021, 73, 924–967. - PubMed
-
- Chistiakov DA, Orekhov AN and Bobryshev YV, Front. Physiol, DOI:10.3389/fphys.2015.00365. - DOI
-
- Phinikaridou A, Andia ME, Protti A, Indermuehle A, Shah A, Smith A, Warley A and Botnar RM, Circulation, 2012, 126, 707–719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

