Synthesis and preclinical evaluation of [11C]EAI045 as a PET tracer for imaging tumors expressing mutated epidermal growth factor receptor
- PMID: 38363422
- PMCID: PMC10873260
- DOI: 10.1186/s13550-024-01078-6
Synthesis and preclinical evaluation of [11C]EAI045 as a PET tracer for imaging tumors expressing mutated epidermal growth factor receptor
Abstract
Background: Mutations in the epidermal growth factor receptor (EGFR) kinase domain are common in non-small cell lung cancer. Conventional tyrosine kinase inhibitors target the mutation site in the ATP binding pocket, thereby inhibiting the receptor's function. However, subsequent treatment resistance mutations in the ATP binding site are common. The EGFR allosteric inhibitor, EAI045, is proposed to have an alternative mechanism of action, disrupting receptor signaling independent of the ATP-binding site. The antibody cetuximab is hypothesized to increase the number of accessible allosteric pockets for EAI045, thus increasing the potency of the inhibitor. This work aimed to gain further knowledge on pharmacokinetics, the EGFR mutation-targeting potential, and the influence of cetuximab on the uptake by radiolabeling EAI045 with carbon-11 and tritium.
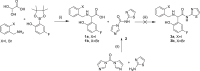
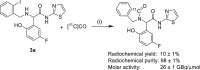
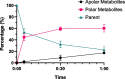
Results: 2-(5-fluoro-2-hydroxyphenyl)-2-((2-iodobenzyl)amino)-N-(thiazol-2-yl)acetamide and 2-(5-fluoro-2-hydroxyphenyl)-N-(5-iodothiazol-2-yl)-2-(1-oxoisoindolin-2-yl)acetamide were synthesized as precursors for the carbon-11 and tritium labeling of EAI045, respectively. [11C]EAI045 was synthesized using [11C]CO in a palladium-catalyzed ring closure in a 10 ± 1% radiochemical yield (decay corrected to end of [11C]CO2 production), > 97% radiochemical purity and 26 ± 1 GBq/µmol molar activity (determined at end of synthesis) in 51 min. [3H]EAI045 was synthesized by a tritium-halogen exchange in a 0.2% radiochemical yield, 98% radiochemical purity, and 763 kBq/nmol molar activity. The ability of [11C]EAI045 to differentiate between L858R/T790M mutated EGFR expressing H1975 xenografts and wild-type EGFR expressing A549 xenografts was evaluated in female nu/nu mice. The uptake was statistically significantly higher in H1975 xenografts compared to A549 xenografts (0.45 ± 0.07%ID/g vs. 0.31 ± 0.10%ID/g, P = 0.0166). The synergy in inhibition between EAI045 and cetuximab was evaluated in vivo and in vitro. While there was some indication that cetuximab influenced the uptake of [3H]EAI045 in vitro, this could not be confirmed in vivo when tumor-bearing mice were administered cetuximab (0.5 mg), 24 h prior to injection of [11C]EAI045.
Conclusions: EAI045 was successfully labeled with tritium and carbon-11, and the in vivo results indicated [11C]EAI045 may be able to distinguish between mutated and non-mutated EGFR in non-small cell lung cancer mouse models. Cetuximab was hypothesized to increase EAI045 uptake; however, no significant effect was observed on the uptake of [11C]EAI045 in vivo or [3H]EAI045 in vitro in H1975 xenografts and cells.
Keywords: EAI045; EGFR; Epidermal growth factor receptor; TKI; Tyrosine kinase inhibitor.
© 2024. The Author(s).
Conflict of interest statement
A.D.W. is editor-in-chief of Nuclear Medicine & Biology.
Figures



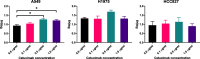




Similar articles
-
Synthesis and preclinical evaluation of two osimertinib isotopologues labeled with carbon-11 as PET tracers targeting the tyrosine kinase domain of the epidermal growth factor receptor.Nucl Med Biol. 2023 May-Jun;120-121:108349. doi: 10.1016/j.nucmedbio.2023.108349. Epub 2023 May 4. Nucl Med Biol. 2023. PMID: 37209556
-
Synthesis and Fundamental Evaluation of Radioiodinated Rociletinib (CO-1686) as a Probe to Lung Cancer with L858R/T790M Mutations of Epidermal Growth Factor Receptor (EGFR).Molecules. 2020 Jun 24;25(12):2914. doi: 10.3390/molecules25122914. Molecules. 2020. PMID: 32599930 Free PMC article.
-
Overcoming EGFR(T790M) and EGFR(C797S) resistance with mutant-selective allosteric inhibitors.Nature. 2016 Jun 2;534(7605):129-32. doi: 10.1038/nature17960. Epub 2016 May 25. Nature. 2016. PMID: 27251290 Free PMC article.
-
Cetuximab: an epidermal growth factor receptor chemeric human-murine monoclonal antibody.Drugs Today (Barc). 2005 Feb;41(2):107-27. doi: 10.1358/dot.2005.41.2.882662. Drugs Today (Barc). 2005. PMID: 15821783 Review.
-
EAI045: The fourth-generation EGFR inhibitor overcoming T790M and C797S resistance.Cancer Lett. 2017 Jan 28;385:51-54. doi: 10.1016/j.canlet.2016.11.008. Epub 2016 Nov 10. Cancer Lett. 2017. PMID: 27840244 Review.
Cited by
-
Design, synthesis, and anticancer assessment of structural analogues of (E)-1-((3,4,5-trimethoxybenzylidene)amino)-4-(3,4,5-trimethoxyphenyl)imidazo[1,2-a]quinoxaline-2-carbonitrile (6b), an imidazo[1,2-a]quinoxaline-based non-covalent EGFR inhibitor.RSC Med Chem. 2024 Jun 20;15(7):2322-2339. doi: 10.1039/d4md00237g. eCollection 2024 Jul 17. RSC Med Chem. 2024. PMID: 39026650 Free PMC article.
-
Efficient mechanochemical synthesis of new fluorinated Schiff bases: a solvent-free, alternative to conventional method with mercury adsorption properties.BMC Chem. 2025 Jul 4;19(1):201. doi: 10.1186/s13065-025-01552-9. BMC Chem. 2025. PMID: 40616138 Free PMC article.
References
-
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

