Risk Factors for Thromboembolic Events in Patients With Dialysis-Dependent CKD: Pooled Analysis of Phase 3 Roxadustat Trials in Japan
- PMID: 38363463
- PMCID: PMC10960897
- DOI: 10.1007/s12325-023-02727-3
Risk Factors for Thromboembolic Events in Patients With Dialysis-Dependent CKD: Pooled Analysis of Phase 3 Roxadustat Trials in Japan
Abstract
Introduction: Thromboembolic events have occurred in clinical trials of roxadustat. This post hoc analysis explored potential factors related to thromboembolic events in dialysis-dependent patients treated with roxadustat in four phase 3 clinical trials in Japan.
Methods: Thromboembolic events with onset before and after week 12 were evaluated. Baseline risk factors for thromboembolic events were investigated by Cox regression analyses. Nested case-control analyses using conditional logistic models with matched pairs of case-control data explored relationships between thromboembolic events and laboratory parameters.
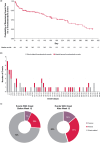
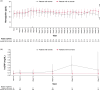
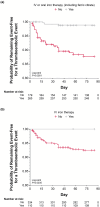
Results: Of the 444 patients, 56 thromboembolic events were observed in 44 patients during ≤ 52 weeks of treatment. The proportion of venous and arterial thromboembolic events gradually increased after week 12. Baseline risk factors included hemodialysis (vs peritoneal dialysis), advanced age (≥ 65 years), shorter dialysis vintage (< 4 months), and history of thromboembolism. The absence of concomitant intravenous or oral iron therapy (including ferric citrate) was associated with thromboembolic events before week 12 (hazard ratio 11.25; 95% confidence interval [CI] 3.36-37.71; vs presence). Case-control analysis revealed that low average transferrin saturation (< 10%; unadjusted odds ratio [OR] 6.25; 95% CI 1.52-25.62; vs ≥ 20%), high average transferrin level (≥ 2.5 g/L; unadjusted OR 4.36; 95% CI 1.23-15.39; vs < 2.0 g/L), and high average roxadustat dose (≥ 150 mg; unadjusted OR 5.95; 95% CI 1.07-33.16; vs < 50 mg) over the previous 8 weeks before the event onset were associated with thromboembolic events after week 12. However, adjustment for iron status extinguished the significant relationship between roxadustat dose and events. Multivariate case-control analysis showed that increased transferrin from baseline (≥ 1.0 g/L; adjusted OR 7.85; 95% CI 1.82-33.90; vs < 0.5 g/dL) and decreased mean corpuscular volume (< - 2 fL; adjusted OR 5.55; 95% CI 1.73-17.83; vs ≥ 0 fL) were associated with increased risk of thromboembolic events.
Conclusion: In addition to established risk factors, iron deficiency may be related to thromboembolic events. Graphical Abstract available for this article.
Trial registration: NCT02780726, NCT02952092, NCT02780141, NCT02779764.
Keywords: Anemia; Chronic kidney disease; Dialysis; Hypoxia-inducible factor prolyl hydroxylase inhibitor; Iron deficiency; Roxadustat; Thromboembolism.
Plain language summary
Roxadustat is an oral medicine that treats anemia in patients with chronic kidney disease (CKD). Thromboembolic events, or blood vessels blocked by a blood clot, have occurred in clinical trials of roxadustat. This study explored potential factors that may be related to thromboembolic events in roxadustat-treated patients with anemia of CKD on dialysis before and after week 12. This study found that hemodialysis (vs peritoneal dialysis), advanced age (older than 65 years), short amount of time on dialysis (less than 4 months), previous history of thromboembolic events, and not receiving iron therapy were risk factors for thromboembolic events before week 12. Iron deficiency and high roxadustat dose were risk factors for thromboembolic events after week 12. When iron status was also considered, we did not find that roxadustat dose was related to thromboembolic events. A different model found that increased levels of transferrin, a protein that transports iron, from baseline and decreased mean corpuscular volume, or smaller red blood cells, increased the risk of thromboembolic events. Patients with anemia of CKD on dialysis may benefit from more intentional monitoring and management of iron while receiving roxadustat.
© 2024. The Author(s).
Conflict of interest statement
Takayuki Hamano has received grants for physician-initiated research, consulting fees, and honoraria for lectures from Astellas Pharma, Inc. Yusuke Yamaguchi is an employee of Astellas Pharma, Inc. Kashia Goto has participated in company stock ownership plan through Astellas Pharma, Inc. Sho Mizokawa is an employee of Astellas Pharma, Inc. Yuichiro Ito is an employee of Astellas Pharma, Inc. Frank Dellanna has nothing to disclose. Jonathan Barratt has received fees for invited lectures from Astellas Pharma Inc. Tadao Akizawa has received personal consulting fees from Astellas Pharma, Inc., Kyowa Kirin, Kissei Pharmaceutical, Ono Pharmaceutical, Fuso Pharmaceutical, Torii Pharmaceutical, GlaxoSmithKline, JT Pharmaceutical, Nipro Corporation, Otsuka, Sanwa Kagaku, and Bayer and has also received personal payment or honoraria for lectures from Astellas Pharma, Inc., Kyowa Kirin, Kissei Pharmaceutical, Ono Pharmaceutical, Fuso Pharmaceutical, Torii Pharmaceutical, Chugai Pharmaceutical, Mitsubishi Tanabe, and Bayer.
Figures

References
-
- Yamamoto H, Nishi S, Tomo T, et al. 2015 Japanese Society for Dialysis Therapy: Guidelines for Renal Anemia in Chronic Kidney Disease. Ren Replace Ther. 2017;3(1).
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

