DLK signaling in axotomized neurons triggers complement activation and loss of upstream synapses
- PMID: 38363678
- PMCID: PMC11088462
- DOI: 10.1016/j.celrep.2024.113801
DLK signaling in axotomized neurons triggers complement activation and loss of upstream synapses
Abstract
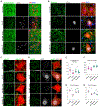
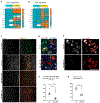
Axotomized spinal motoneurons (MNs) lose presynaptic inputs following peripheral nerve injury; however, the cellular mechanisms that lead to this form of synapse loss are currently unknown. Here, we delineate a critical role for neuronal kinase dual leucine zipper kinase (DLK)/MAP3K12, which becomes activated in axotomized neurons. Studies with conditional knockout mice indicate that DLK signaling activation in injured MNs triggers the induction of phagocytic microglia and synapse loss. Aspects of the DLK-regulated response include expression of C1q first from the axotomized MN and then later in surrounding microglia, which subsequently phagocytose presynaptic components of upstream synapses. Pharmacological ablation of microglia inhibits the loss of cholinergic C boutons from axotomized MNs. Together, the observations implicate a neuronal mechanism, governed by the DLK, in the induction of inflammation and the removal of synapses.
Keywords: CP: Neuroscience; axon damage; axonal regeneration; c1q; microglia; motoneuron; peripheral nerve; phagocytosis; plasticity; stress response; synapse stripping.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

