Chiral Induced Spin Selectivity
- PMID: 38364021
- PMCID: PMC10906005
- DOI: 10.1021/acs.chemrev.3c00661
Chiral Induced Spin Selectivity
Abstract
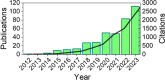
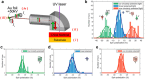
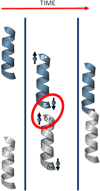
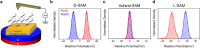
Since the initial landmark study on the chiral induced spin selectivity (CISS) effect in 1999, considerable experimental and theoretical efforts have been made to understand the physical underpinnings and mechanistic features of this interesting phenomenon. As first formulated, the CISS effect refers to the innate ability of chiral materials to act as spin filters for electron transport; however, more recent experiments demonstrate that displacement currents arising from charge polarization of chiral molecules lead to spin polarization without the need for net charge flow. With its identification of a fundamental connection between chiral symmetry and electron spin in molecules and materials, CISS promises profound and ubiquitous implications for existing technologies and new approaches to answering age old questions, such as the homochiral nature of life. This review begins with a discussion of the different methods for measuring CISS and then provides a comprehensive overview of molecules and materials known to exhibit CISS-based phenomena before proceeding to identify structure-property relations and to delineate the leading theoretical models for the CISS effect. Next, it identifies some implications of CISS in physics, chemistry, and biology. The discussion ends with a critical assessment of the CISS field and some comments on its future outlook.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




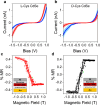



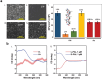
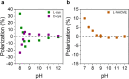
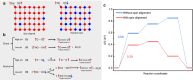

Similar articles
-
Chirality-Induced Spin Selectivity in Composite Materials: A Device Perspective.Acc Chem Res. 2024 May 21;57(10):1478-1487. doi: 10.1021/acs.accounts.4c00077. Epub 2024 Apr 30. Acc Chem Res. 2024. PMID: 38687873 Free PMC article.
-
Chirality induced spin selectivity in electron transport investigated by scanning probe microscopy.J Phys Condens Matter. 2025 Jan 13;37(11). doi: 10.1088/1361-648X/ada478. J Phys Condens Matter. 2025. PMID: 39740349 Review.
-
Chiral-induced spin selectivity in biomolecules, hybrid organic-inorganic perovskites and inorganic materials: a comprehensive review on recent progress.Mater Horiz. 2023 Jun 6;10(6):1924-1955. doi: 10.1039/d3mh00024a. Mater Horiz. 2023. PMID: 36989068 Review.
-
Chiral Induced Spin Selectivity Gives a New Twist on Spin-Control in Chemistry.Acc Chem Res. 2020 Nov 17;53(11):2659-2667. doi: 10.1021/acs.accounts.0c00485. Epub 2020 Oct 12. Acc Chem Res. 2020. PMID: 33044813 Free PMC article.
-
Efficient Transfer of Chirality in Complex Hybrid Materials and Impact on Chirality-induced Spin Selectivity.Chem Mater. 2024 Nov 20;36(23):11449-11461. doi: 10.1021/acs.chemmater.4c02108. eCollection 2024 Dec 10. Chem Mater. 2024. PMID: 39678932 Free PMC article.
Cited by
-
Current Induced Spin-Polarization in Chiral Molecules.J Phys Chem Lett. 2024 Jun 20;15(24):6370-6374. doi: 10.1021/acs.jpclett.4c01362. Epub 2024 Jun 10. J Phys Chem Lett. 2024. PMID: 38857512 Free PMC article.
-
Homochiral Carboxylate-Anchored Truxene Tripods: Design, Synthesis, and Monolayer Formation on Ag(111).Chemistry. 2025 Apr 4;31(20):e202404750. doi: 10.1002/chem.202404750. Epub 2025 Feb 21. Chemistry. 2025. PMID: 39963079 Free PMC article.
-
Helicenes with Four Helical Turns: Dimerization of [13]Helicenes to [27]Helicenoids.Angew Chem Int Ed Engl. 2025 Jul;64(29):e202506328. doi: 10.1002/anie.202506328. Epub 2025 May 27. Angew Chem Int Ed Engl. 2025. PMID: 40420655 Free PMC article.
-
Chirality in nanomaterials.Sci Rep. 2024 Nov 1;14(1):26268. doi: 10.1038/s41598-024-77887-5. Sci Rep. 2024. PMID: 39487203 Free PMC article.
-
Precision Intercalation of Organic Molecules in 2D Layered Materials: From Interface Chemistry to Low-Dimensional Physics.Precis Chem. 2025 Jan 10;3(2):51-71. doi: 10.1021/prechem.4c00084. eCollection 2025 Feb 24. Precis Chem. 2025. PMID: 40018453 Free PMC article. Review.
References
-
- MacDermott A.J.; Barron L.D.; Brack A.; Buhse T.; Drake A.F.; Emery R.; Gottarelli G.; Greenberg J.M.; Haberle R.; Hegstrom R.A.; Hobbs K.; Kondepudi D.K.; McKay C.; Moorbath S.; Raulin F.; Sandford M.; Schwartzman D.W.; Thiemann W.H.-P.; Tranter G.E.; Zarnecki J.C. Homochirality as the Signature of Life: the SETH Cigar. Planet. Space Sci. 1996, 44, 1441–1446. 10.1016/S0032-0633(96)00057-8. - DOI - PubMed
-
- Meierhenrich U.Amino Acids and the Asymmetry of Life: Caught in the Act of Formation; Springer. 2008.
-
- Lee T. D.; Yang C. N. Question of Parity Conservation in Weak Interactions. Phys. Rev. 1956, 104, 254–258. 10.1103/PhysRev.104.254. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

