The influence of voxelotor on cerebral blood flow and oxygen extraction in pediatric sickle cell disease
- PMID: 38364110
- PMCID: PMC11443564
- DOI: 10.1182/blood.2023022011
The influence of voxelotor on cerebral blood flow and oxygen extraction in pediatric sickle cell disease
Abstract
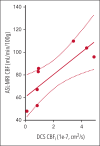
Voxelotor is an inhibitor of sickle hemoglobin polymerization that is used to treat sickle cell disease. Although voxelotor has been shown to improve anemia, the clinical benefit on the brain remains to be determined. This study quantified the cerebral hemodynamic effects of voxelotor in children with sickle cell anemia (SCA) using noninvasive diffuse optical spectroscopies. Specifically, frequency-domain near-infrared spectroscopy combined with diffuse correlation spectroscopy were used to noninvasively assess regional oxygen extraction fraction (OEF), cerebral blood volume, and an index of cerebral blood flow (CBFi). Estimates of CBFi were first validated against arterial spin-labeled magnetic resonance imaging (ASL-MRI) in 8 children with SCA aged 8 to 18 years. CBFi was significantly positively correlated with ASL-MRI-measured blood flow (R2 = 0.651; P = .015). Next, a single-center, open-label pilot study was completed in 8 children with SCA aged 4 to 17 years on voxelotor, monitored before treatment initiation and at 4, 8, and 12 weeks (NCT05018728). By 4 weeks, both OEF and CBFi significantly decreased, and these decreases persisted to 12 weeks (both P < .05). Decreases in CBFi were significantly correlated with increases in blood hemoglobin (Hb) concentration (P = .025), whereas the correlation between decreases in OEF and increases in Hb trended toward significance (P = .12). Given that previous work has shown that oxygen extraction and blood flow are elevated in pediatric SCA compared with controls, these results suggest that voxelotor may reduce cerebral hemodynamic impairments. This trial was registered at www.ClinicalTrials.gov as #NCT05018728.
© 2024 American Society of Hematology. Published by Elsevier Inc. All rights are reserved, including those for text and data mining, AI training, and similar technologies.
Conflict of interest statement
Conflict-of-interest disclosure: During the conduct of this study, R.C.B. was employed at Emory University. R.C.B. is currently employed by and has equity ownership with Pfizer; is a former employee of Global Blood Therapeutics; has received consultant fees from Global Blood Therapeutics, Imara, and Novartis; and has received research funding from Forma Therapeutics, Global Blood Therapeutics, Imara, Novartis, and Pfizer. E.M.B. has received research funding from Pfizer, Global Blood Therapeutics, and Novartis. A.G.-Y. has received research funding from Pfizer, Global Blood Therapeutics, and Novartis. The remaining authors declare no competing financial interests.
The current affiliation of R.C.B. is Pfizer, Atlanta, GA.
Figures

Comment in
-
Oxygen tug-of-war, voxelotor, and the brain.Blood. 2024 May 23;143(21):2113-2114. doi: 10.1182/blood.2024024190. Blood. 2024. PMID: 38780920 No abstract available.
References
-
- Runge A, Brazel D, Pakbaz Z. Stroke in sickle cell disease and the promise of recent disease modifying agents. J Neurol Sci. 2022;442 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

