Towards scaling up the sonochemical synthesis of Pt-nanocatalysts
- PMID: 38364482
- PMCID: PMC10878992
- DOI: 10.1016/j.ultsonch.2024.106794
Towards scaling up the sonochemical synthesis of Pt-nanocatalysts
Abstract
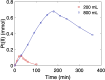
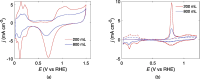
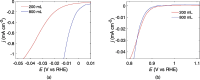
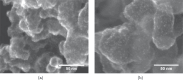
Large scale production of electrocatalysts for electrochemical energy conversion devices such as proton exchange membrane fuel cells must be developed to reduce their cost. The current chemical reduction methods used for this synthesis suffer from problems with achieving similar particle properties such as particle size and catalytic activity when scaling up the volume or the precursor concentration. The continuous production of reducing agents through the sonochemical synthesis method could help maintain the reducing conditions (and also the particle properties) upon increasing the reactor volume. In this work we demonstrate that the reducing conditions of Pt-nanoparticles are indeed maintained when the reactor volume is increased from 200 mL to 800 mL. Similar particle sizes, 2.1(0.3) nm at 200 mL and 2.3(0.4) nm at 800 mL, and catalytic activities towards the oxygen reduction reaction (ORR) are maintained as well. The reducing conditions were assessed through TiOSO4 dosimetry, sonochemiluminesence imaging, acoustic power measurements, and Pt(II) reduction rate measurements. Cyclic voltammetry, CO-stripping, hydrogen evolution measurements, ORR measurements, and electron microscopy were used to evaluate the catalytic activity and particle size. The similar particle properties displayed from the two reactor volumes suggest that the sonochemical synthesis of Pt-nanoparticles is suitable for large scale production.
Keywords: Electrocatalysts; Fuel cells; Nanoparticle synthesis; ORR; Pt; Scale-up; Sonochemistry.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Pollet B.G., Kocha S.S., Staffell I. Current status of automotive fuel cells for sustainable transport. Current Opinion in Electrochemistry 16. Electrochem. Mater. Eng. Sensors Biosensors. 2019:90–95. issn: 2451-9103.
-
- Wang J. Barriers of scaling-up fuel cells: Cost, durability and reliability. Energy. 2015;80:509–521. issn: 0360-5442.
-
- Shao M., Chang Q., Dodelet J.-P., Chenitz R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016;116:3594–3657. - PubMed
-
- Stephens I.E.L., Bondarenko A.S., Grønbjerg U., Rossmeisl J., Chorkendorff I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 2012;5:6744–6762. 5.
-
- Gasteiger H.A., Kocha S.S., Sompalli B., Wagner F.T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B: Environ. 2005;56:9–35. Fuel processing and PEM Fuel Cells: advanced cataysts, adsorbents and electrocatalysts.
LinkOut - more resources
Full Text Sources

