FLASH Proton Radiation Therapy Mitigates Inflammatory and Fibrotic Pathways and Preserves Cardiac Function in a Preclinical Mouse Model of Radiation-Induced Heart Disease
- PMID: 38364948
- PMCID: PMC11209795
- DOI: 10.1016/j.ijrobp.2024.01.224
FLASH Proton Radiation Therapy Mitigates Inflammatory and Fibrotic Pathways and Preserves Cardiac Function in a Preclinical Mouse Model of Radiation-Induced Heart Disease
Abstract
Purpose: Studies during the past 9 years suggest that delivering radiation at dose rates exceeding 40 Gy/s, known as "FLASH" radiation therapy, enhances the therapeutic index of radiation therapy (RT) by decreasing normal tissue damage while maintaining tumor response compared with conventional (or standard) RT. This study demonstrates the cardioprotective benefits of FLASH proton RT (F-PRT) compared with standard (conventional) proton RT (S-PRT), as evidenced by reduced acute and chronic cardiac toxicities.
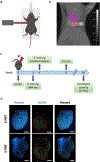
Methods and materials: Mice were imaged using cone beam computed tomography to precisely determine the heart's apex as the beam isocenter. Irradiation was conducted using a shoot-through technique with a 5-mm diameter circular collimator. Bulk RNA-sequencing was performed on nonirradiated samples, as well as apexes treated with F-PRT or S-PRT, at 2 weeks after a single 40 Gy dose. Inflammatory responses were assessed through multiplex cytokine/chemokine microbead assay and immunofluorescence analyses. Levels of perivascular fibrosis were quantified using Masson's Trichrome and Picrosirius red staining. Additionally, cardiac tissue functionality was evaluated by 2-dimensional echocardiograms at 8- and 30-weeks post-PRT.
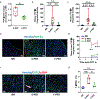
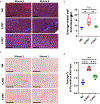
Results: Radiation damage was specifically localized to the heart's apex. RNA profiling of cardiac tissues treated with PRT revealed that S-PRT uniquely upregulated pathways associated with DNA damage response, induction of tumor necrosis factor superfamily, and inflammatory response, and F-PRT primarily affected cytoplasmic translation, mitochondrion organization, and adenosine triphosphate synthesis. Notably, F-PRT led to a milder inflammatory response, accompanied by significantly attenuated changes in transforming growth factor β1 and α smooth muscle actin levels. Critically, F-PRT decreased collagen deposition and better preserved cardiac functionality compared with S-PRT.
Conclusions: This study demonstrated that F-PRT reduces the induction of an inflammatory environment with lower expression of inflammatory cytokines and profibrotic factors. Importantly, the results indicate that F-PRT better preserves cardiac functionality, as confirmed by echocardiography analysis, while also mitigating the development of long-term fibrosis.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Delaney G, Jacob S, Featherstone C, Barton M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005;104:1129–1137. - PubMed
-
- Ghobadi G, van der Veen S, Bartelds B, et al. Physiological interaction of heart and lung in thoracic irradiation. Int J Radiat Oncol Biol Phys 2012;84:e639–e646. - PubMed
-
- Darby SC, Ewertz M, Hall P. Ischemic heart disease after breast cancer radiotherapy. N Engl J Med 2013;368:2527. - PubMed
-
- Galper SL, Yu JB, Mauch PM, et al. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood 2011;117:412–418. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

