Anaerobic hexadecane degradation by a thermophilic Hadarchaeon from Guaymas Basin
- PMID: 38365230
- PMCID: PMC10811742
- DOI: 10.1093/ismejo/wrad004
Anaerobic hexadecane degradation by a thermophilic Hadarchaeon from Guaymas Basin
Abstract
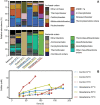
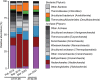
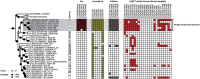
Hadarchaeota inhabit subsurface and hydrothermally heated environments, but previous to this study, they had not been cultured. Based on metagenome-assembled genomes, most Hadarchaeota are heterotrophs that grow on sugars and amino acids, or oxidize carbon monoxide or reduce nitrite to ammonium. A few other metagenome-assembled genomes encode alkyl-coenzyme M reductases (Acrs), β-oxidation, and Wood-Ljungdahl pathways, pointing toward multicarbon alkane metabolism. To identify the organisms involved in thermophilic oil degradation, we established anaerobic sulfate-reducing hexadecane-degrading cultures from hydrothermally heated sediments of the Guaymas Basin. Cultures at 70°C were enriched in one Hadarchaeon that we propose as Candidatus Cerberiarchaeum oleivorans. Genomic and chemical analyses indicate that Ca. C. oleivorans uses an Acr to activate hexadecane to hexadecyl-coenzyme M. A β-oxidation pathway and a tetrahydromethanopterin methyl branch Wood-Ljungdahl (mWL) pathway allow the complete oxidation of hexadecane to CO2. Our results suggest a syntrophic lifestyle with sulfate reducers, as Ca. C. oleivorans lacks a sulfate respiration pathway. Comparative genomics show that Acr, mWL, and β-oxidation are restricted to one family of Hadarchaeota, which we propose as Ca. Cerberiarchaeaceae. Phylogenetic analyses further indicate that the mWL pathway is basal to all Hadarchaeota. By contrast, the carbon monoxide dehydrogenase/acetyl-coenzyme A synthase complex in Ca. Cerberiarchaeaceae was horizontally acquired from Bathyarchaeia. The Acr and β-oxidation genes of Ca. Cerberiarchaeaceae are highly similar to those of other alkane-oxidizing archaea such as Ca. Methanoliparia and Ca. Helarchaeales. Our results support the use of Acrs in the degradation of petroleum alkanes and suggest a role of Hadarchaeota in oil-rich environments.
Keywords: Archaea; alkanes; anaerobic metabolism; evolution; hydrocarbons.
© The Author(s) 2024. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Thauer RK, Kaster AK, Seedorf Het al. . Methanogenic archaea: ecologically relevant differences in energy conservation. Nat Rev Microbiol 2008;6:579–91. - PubMed
-
- Shima S, Krueger M, Weinert Tet al. . Structure of a methyl-coenzyme M reductase from Black Sea mats that oxidize methane anaerobically. Nature 2012;481:98–101. - PubMed
-
- Hallam SJ, Putnam N, Preston CMet al. . Reverse methanogenesis: testing the hypothesis withenvironmental genomics. Science 2004;305:1457–62. - PubMed
-
- Laso-Pérez R, Wegener G, Knittel Ket al. . Thermophilic archaea activate butane via alkyl-coenzyme M formation. Nature 2016;539:396–401. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

