Comparing one dose of HPV vaccine in girls aged 9-14 years in Tanzania (DoRIS) with one dose in young women aged 15-20 years in Kenya (KEN SHE): an immunobridging analysis of randomised controlled trials
- PMID: 38365419
- PMCID: PMC10882205
- DOI: 10.1016/S2214-109X(23)00586-7
Comparing one dose of HPV vaccine in girls aged 9-14 years in Tanzania (DoRIS) with one dose in young women aged 15-20 years in Kenya (KEN SHE): an immunobridging analysis of randomised controlled trials
Abstract
Background: The first randomised controlled trial of single-dose human papillomavirus (HPV) vaccine efficacy, the Kenya single-dose HPV-vaccine efficacy (KEN SHE) trial, showed greater than 97% efficacy against persistent HPV16 and HPV18 infection at 36 months among women in Kenya. We compared antibody responses after one dose of HPV vaccine in the Dose Reduction Immunobridging and Safety Study (DoRIS), the first randomised trial of the single- dose regimen in girls aged 9-14 years, the target age range for vaccination, with those after one dose of the same vaccine in KEN SHE.
Methods: In the DoRIS trial, 930 girls aged 9-14 years in Tanzania were randomly assigned to one, two, or three doses of the 2-valent vaccine (Cervarix) or the 9-valent vaccine (Gardasil-9). The proportion seroconverting and geometric mean concentrations (GMCs) at month 24 after one dose were compared with those in women aged 15-20 years who were randomly assigned to one dose of the same vaccines at the same timepoint in KEN SHE. Batched samples were tested together by virus-like particle ELISA for HPV16 and HPV18 IgG antibodies. Non-inferiority of GMC ratios (DoRIS trial:KEN SHE) was predefined as a lower bound of the 95% CI less than 0·50.
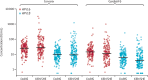
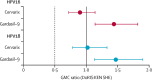
Findings: Month 24 HPV16 and HPV18 antibody GMCs in DoRIS were similar or higher than those in KEN SHE. 2-valent GMC ratios were 0·90 (95% CI 0·72-1·14) for HPV16 and 1·02 (0·78-1·33) for HPV18. 9-valent GMC ratios were 1·44 (95% CI 1·14-1·82) and 1·47 (1·13-1·90), respectively. Non-inferiority of antibody GMCs and seropositivity was met for HPV16 and HPV18 for both vaccines.
Interpretation: HPV16 and HPV18 immune responses in young girls 24 months after a single dose of 2-valent or 9-valent HPV vaccine were comparable to those in young women who were randomly assigned to a single dose of the same vaccines and in whom efficacy had been shown. A single dose of HPV vaccine, when given to girls in the target age range for vaccination, induces immune responses that could be effective against persistent HPV16 and HPV18 infection at least two years after vaccination.
Funding: The UK Department of Health and Social Care, the Foreign, Commonwealth, & Development Office, the Global Challenges Research Fund, the UK Medical Research Council and Wellcome Trust Joint Global Health Trials scheme, the Bill and Melinda Gates Foundation, the US National Cancer Institute; the US National Institutes of Health, and the Francis and Dorothea Reed Endowed Chair in Infectious Diseases.
Translation: For the KiSwahili translation of the abstract see Supplementary Materials section.
Copyright © 2024 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests KB reports grants from the Bill & Melinda Gates Foundation during the conduct of the DoRIS trial, and a grant and vaccine donations from Merck Pharmaceuticals outside the submitted work. DW-J reports grants from the Gates Foundation and the UK Research and Innovation Medical Research Council during the DoRIS trial, and a grant and vaccine donations from Merck outside the submitted work. HW reports grants from the Gates Foundation and the UK Research and Innovation Medical Research Council during the DoRIS trial, and vaccine donations from Merck outside the submitted work. JC reports grant support from the Gates Foundation and the UK Research and Innovation Medical Research Council during the DoRIS trial. RJH reports a grant from the UK Research and Innovation Medical Research Council during the DoRIS trial. RVB reports grants from the Gates Foundation during the conduct of the KEN SHE trial, and data monitoring committee honorarium from Gilead Sciences and manuscript and abstract writing support from Regeneron Pharmaceuticals outside the submitted work. NRM reports grant support from Merck Pharmaceuticals outside the submitted work. DAG reports grants from the Gates Foundation during the conduct of the KEN SHE trial, and grants and personal fees from Merck outside the submitted work. EAB reports grants from the National Institutes of Health, the Centers for Disease Control and Prevention, and the European and Developing Countries Clinical Trials Partnership during the conduct of the KEN SHE trial; and personal fees from Gilead Sciences and personal fees from Merck outside the submitted work. All other authors declare no competing interests.
Figures
Comment in
-
Single dose HPV vaccine in achieving global cervical cancer elimination.Lancet Glob Health. 2024 Mar;12(3):e360-e361. doi: 10.1016/S2214-109X(24)00009-3. Lancet Glob Health. 2024. PMID: 38365404 No abstract available.
References
-
- WHO Global Strategy to accelerate the elimination of cervical cancer as a public health problem. Nov 17, 2020. https://www.who.int/publications/i/item/9789240014107
-
- WHO Human papillomavirus (HPV) vaccination coverage. https://immunizationdata.who.int/pages/coverage/hpv.html
-
- Gallagher KE, LaMontagne DS, Watson-Jones D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine. 2018;36(32 Pt A):4761–4767. - PubMed
-
- Sankaranarayanan R, Joshi S, Muwonge R, et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine. 2018;36(32 Pt A):4783–4791. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

