Neoadjuvant cobimetinib and atezolizumab with or without vemurafenib for high-risk operable Stage III melanoma: the Phase II NeoACTIVATE trial
- PMID: 38365756
- PMCID: PMC10873383
- DOI: 10.1038/s41467-024-45798-8
Neoadjuvant cobimetinib and atezolizumab with or without vemurafenib for high-risk operable Stage III melanoma: the Phase II NeoACTIVATE trial
Abstract
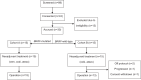
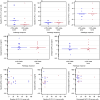
Both targeted therapies and immunotherapies provide benefit in resected Stage III melanoma. We hypothesized that the combination of targeted and immunotherapy given prior to therapeutic lymph node dissection (TLND) would be tolerable and drive robust pathologic responses. In NeoACTIVATE (NCT03554083), a Phase II trial, patients with clinically evident resectable Stage III melanoma received either 12 weeks of neoadjuvant vemurafenib, cobimetinib, and atezolizumab (BRAF-mutated, Cohort A, n = 15), or cobimetinib and atezolizumab (BRAF-wild-type, Cohort B, n = 15) followed by TLND and 24 weeks of adjuvant atezolizumab. Here, we report outcomes from the neoadjuvant portion of the trial. Based on intent to treat analysis, pathologic response (≤50% viable tumor) and major pathologic response (complete or near-complete, ≤10% viable tumor) were observed in 86.7% and 66.7% of BRAF-mutated and 53.3% and 33.3% of BRAF-wild-type patients, respectively (primary outcome); these exceeded pre-specified benchmarks of 50% and 30% for major pathologic response. Grade 3 and higher toxicities, primarily dermatologic, occurred in 63% during neoadjuvant treatment (secondary outcome). No surgical delays nor progression to regional unresectability occurred (secondary outcome). Peripheral blood CD8 + TCM cell expansion associated with favorable pathologic responses (exploratory outcome).
© 2024. The Author(s).
Conflict of interest statement
T.J.H.: Research support—Genentech, SkylineDX BV; R.R.M.: Research support—Bristol-Myers Squibb, GSK; L.A.K.: Advisory Board—Immunocore; R.S.D.: Research support—Bristol-Myers Squibb; S.N.M.: Research support—Bristol-Myers Squibb, Sorrento Therapeutics; Intellectual Property: Sorrento Therapeutics; A.D.: Honoraria—Intellisphere, Roche/Genentech; Advisory Board—TP Therapeutics, Guardant Health, AnHeart Therapeutics, ChromaCode; Clinical trial support—Syntrix Pharmaceuticals, Novartis, Merck, AnHeart Therapeutics, Sorrento Therapeutics, Guardant, Philogen, AstraZeneca; M.A.P.: Research funding—Intuitive Surgical; Honoraria—Kubtec Medical Imaging; D.L.P.: Advisory Board and Equity Interest—InSitu Biologics; Advisory Board—Medivis; M.S.B.: Research support—Alkermes, Bristol-Myers Squibb, Genentech, Merck, nFerence, Pharmacylclics, Regeneron, Sorrento Therapeutics, TILT Biotherapeutics, Transgene, Viewpoint Molecular Therapeutics; Consultant/Scientific Advisory Board—Sorrento Therapeutics, TILT Biotherapeutics, Viewpoint Molecular Therapeutics All other authors have no competing interests to declare.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

