Quantum measurement enables single biomarker sensitivity in flow cytometry
- PMID: 38365797
- PMCID: PMC10873388
- DOI: 10.1038/s41598-023-49145-7
Quantum measurement enables single biomarker sensitivity in flow cytometry
Abstract
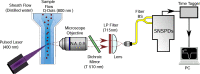
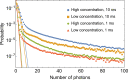
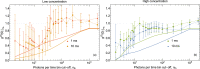
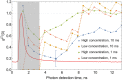
We present the first unambiguous experimental method enabling single-fluorophore sensitivity in a flow cytometer using quantum properties of single-photon emitters. We use a quantum measurement based on the second-order coherence function to prove that the optical signal is produced by individual biomarkers traversing the interrogation volume of the flow cytometer from the first principles. This observation enables the use of the quantum toolbox for rapid detection, enumeration, and sorting of single fluorophores in large cell populations as well as a 'photons-to-moles' calibration of this measurement modality.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply 2024.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Shapiro HM. Practical Flow Cytometry. Wiley; 2005.
-
- Campana D, Coustan-Smith E. Detection of minimal residual disease in acute leukemia by flow cytometry. Cytometry J. Int. Soc. Analyt. Cytol. 1999;38:139. - PubMed
-
- Craig FE, Foon KA. Flow cytometric immunophenotyping for hematologic neoplasms. Blood J. Am. Soc. Hematol. 2008;111:3941. - PubMed
-
- Van Dongen J, Lhermitte L, Böttcher S, Almeida J, Van Der Velden V, Flores-Montero J, Rawstron A, Asnafi V, Lecrevisse Q, Lucio P, et al. Euroflow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26:1908. doi: 10.1038/leu.2012.120. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

