Biocompatibility and sub-chronic toxicity studies of phlorotannin/polycaprolactone coated trachea tube for advancing medical device applications
- PMID: 38365854
- PMCID: PMC10873353
- DOI: 10.1038/s41598-024-54684-8
Biocompatibility and sub-chronic toxicity studies of phlorotannin/polycaprolactone coated trachea tube for advancing medical device applications
Abstract
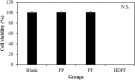
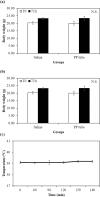
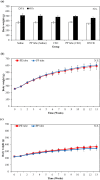
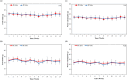
The phlorotannin-polycaprolactone-coated endotracheal tube (PP tube) has been developed with the aim of preventing tracheal stenosis that can result from endotracheal intubation, a factor that can lead to a serious airway obstruction. Its preventive efficacy has been assessed through both in vitro and in vivo investigations. However, there is a lack of studies concerning its biocompatibility and sub-chronic toxicity in animal models, a crucial factor to ensure the safety of its usage as a functional endotracheal tube. Thus, this study aimed to evaluate the biocompatibility and sub-chronic (13 weeks) toxicity of the PP tube through L929 cell line and diverse in vivo models. The cytotoxicity testing was performed using the extracts of PP tube on L929 cells for 72 h. Furthermore, other tests conducted on animal models, including ICR mice (acute systemic toxicity), New Zealand white rabbit (intradermal reactivity and pyrogen tests), guinea pig (maximization sensitization), and Sprague Dawley rats (sub-chronic toxicity). In both biocompatibility and sub-chronic toxicity analyses, no significant adverse effects are observed in the groups exposed to the PP tube, when compared to control group. Altogether, the findings suggested that the PP tube exhibits relative non-toxic and safety, supporting its suitability for clinical usage. However, extended periods of intubation may produce mild irritant responses, highlighting the clinical caution of limiting intubation duration to less than 13 weeks.
Keywords: Biocompatibility; Endotracheal tube; Medical device; Phlorotannin; Sub-chronic toxicity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Fabrication and biological activity of polycaprolactone/phlorotannin endotracheal tube to prevent tracheal stenosis: An in vitro and in vivo study.J Biomed Mater Res B Appl Biomater. 2020 Apr;108(3):1046-1056. doi: 10.1002/jbm.b.34456. Epub 2019 Aug 7. J Biomed Mater Res B Appl Biomater. 2020. PMID: 31392823
-
Rabbit model of tracheal stenosis induced by prolonged endotracheal intubation using a segmented tube.Int J Pediatr Otorhinolaryngol. 2015 Dec;79(12):2384-8. doi: 10.1016/j.ijporl.2015.10.049. Epub 2015 Nov 3. Int J Pediatr Otorhinolaryngol. 2015. PMID: 26586243
-
Local Delivery of Mometasone Furoate from an Eluting Endotracheal Tube Reduces Airway Morbidity Following Long-Term Animal Intubation.ACS Appl Bio Mater. 2021 May 17;4(5):4131-4139. doi: 10.1021/acsabm.0c01526. Epub 2021 Apr 12. ACS Appl Bio Mater. 2021. PMID: 35006827
-
Verification of endotracheal tube position.Anesthesiol Clin North Am. 2001 Dec;19(4):813-39. doi: 10.1016/s0889-8537(01)80012-2. Anesthesiol Clin North Am. 2001. PMID: 11778382 Review.
-
Airway injury after tracheotomy and endotracheal intubation.Surg Clin North Am. 1991 Dec;71(6):1211-30. doi: 10.1016/s0039-6109(16)45586-6. Surg Clin North Am. 1991. PMID: 1948570 Review.
Cited by
-
Multifunctional Microneedle Patch with Diphlorethohydroxycarmalol for Potential Wound Dressing.Tissue Eng Regen Med. 2024 Oct;21(7):1007-1019. doi: 10.1007/s13770-024-00655-z. Epub 2024 Jun 14. Tissue Eng Regen Med. 2024. PMID: 38877361 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

