The serum thioredoxin-1 levels are not associated with bronchopulmonary dysplasia and retinopathy of prematurity
- PMID: 38365875
- PMCID: PMC11521992
- DOI: 10.1038/s41390-024-03078-7
The serum thioredoxin-1 levels are not associated with bronchopulmonary dysplasia and retinopathy of prematurity
Abstract
Background: We hypothesized that the serum TRX-1 in extremely preterm infants (EPIs) after birth was associated with the development of severe bronchopulmonary dysplasia (BPD) and retinopathy of prematurity (ROP).
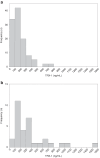
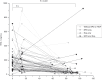
Methods: This single-centered retrospective study enrolled EPIs treated at our institution. Serum TRX-1 concentrations of the residual samples taken on admission, day 10-20 of life, and 36-40 weeks of postmenstrual age (PMA) were measured with an enzyme-linked immunosorbent assay.
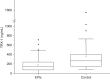
Results: The serum TRX-1 levels on admission were not different between the severe BPD (n = 46) and non-severe BPD groups (n = 67): [median (interquartile range) 147 (73.0-231) vs. 164 (80.5-248) ng/mL] (P = 0.57). These had no significant difference between the severe ROP (n = 47) and non-severe ROP groups (n = 66): [164 (71.3-237) vs. 150 (80.9-250) ng/mL] (P = 0.93). The TRX-1 levels at 10-20 days of life and 36-40 weeks of PMA also had no association with the development of severe BPD and ROP.
Conclusion: The serum TRX-1 levels after birth are not predictive of severe BPD and ROP.
Impact: Serum thioredoxin-1 levels in extremely preterm infants on the day of birth are lower than those in term or near-term infants hospitalized for transient tachypnea of the newborn. In extremely preterm infants, the serum thioredoxin-1 levels on the day of birth, at 10-20 days of life, and at postmenstrual age of 36-40 weeks were not associated with severe bronchopulmonary dysplasia and retinopathy of prematurity. The thioredoxin system is under development in extremely preterm infants; however, the serum thioredoxin-1 level is not predictive for severe bronchopulmonary dysplasia and retinopathy of prematurity.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Comment in
-
Thioredoxin: an antioxidant, a therapeutic target and a possible biomarker.Pediatr Res. 2024 Oct;96(5):1117-1119. doi: 10.1038/s41390-024-03370-6. Epub 2024 Jun 28. Pediatr Res. 2024. PMID: 38942889 Free PMC article. No abstract available.
Similar articles
-
Postnatal serum insulin-like growth factor I deficiency is associated with retinopathy of prematurity and other complications of premature birth.Pediatrics. 2003 Nov;112(5):1016-20. doi: 10.1542/peds.112.5.1016. Pediatrics. 2003. PMID: 14595040
-
Risk factors for retinopathy of prematurity among preterm infants with bronchopulmonary dysplasia.J Matern Fetal Neonatal Med. 2025 Dec;38(1):2497058. doi: 10.1080/14767058.2025.2497058. Epub 2025 May 13. J Matern Fetal Neonatal Med. 2025. PMID: 40360447
-
Neonatal hematological parameters and the risk of moderate-severe bronchopulmonary dysplasia in extremely premature infants.BMC Pediatr. 2019 Apr 30;19(1):138. doi: 10.1186/s12887-019-1515-6. BMC Pediatr. 2019. PMID: 31039810 Free PMC article.
-
Inositol in preterm infants at risk for or having respiratory distress syndrome.Cochrane Database Syst Rev. 2019 Jul 8;7(7):CD000366. doi: 10.1002/14651858.CD000366.pub4. Cochrane Database Syst Rev. 2019. PMID: 31283839 Free PMC article.
-
Vitamin E and preterm infants.Free Radic Biol Med. 2022 Feb 20;180:13-32. doi: 10.1016/j.freeradbiomed.2021.11.037. Epub 2021 Dec 4. Free Radic Biol Med. 2022. PMID: 34871765 Review.
References
-
- Soothill, P. W., Nicolaides, K. H., Rodeck, C. H. & Gamsu, H. Blood gases and acid-base status of the human second-trimester fetus. Obstet. Gynecol.68, 173–176 (1986). - PubMed
-
- Soothill, P. W., Nicolaides, K. H., Rodeck, C. H. & Campbell, S. Effect of gestational age on fetal and intervillous blood gas and acid-base values in human pregnancy. Fetal Ther.1, 168–175 (1986). - PubMed
-
- Lorente-Pozo, S. et al. Oxygen in the neonatal period: Oxidative stress, oxygen load and epigenetic changes. Semin. Fetal Neonatal Med.25, 101090 (2020). - PubMed
-
- He, L. et al. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem.44, 532–553 (2017). - PubMed
-
- Davis, J. M. & Auten, R. L. Maturation of the antioxidant system and the effects on preterm birth. Semin. Fetal Neonatal. Med.15, 191–195 (2010). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

