Anticoagulation with osocimab in patients with kidney failure undergoing hemodialysis: a randomized phase 2 trial
- PMID: 38365952
- PMCID: PMC10878964
- DOI: 10.1038/s41591-023-02794-7
Anticoagulation with osocimab in patients with kidney failure undergoing hemodialysis: a randomized phase 2 trial
Abstract
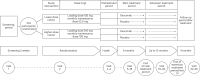
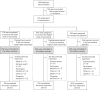
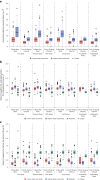
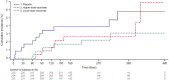
Individuals with kidney failure undergoing hemodialysis are at elevated risk for thromboembolic events. Factor (F) XI, which is in the intrinsic pathway of coagulation, is emerging as an attractive target for new anticoagulants that may be safer than existing agents. Osocimab-an inhibitory FXIa antibody-is a potential treatment option for such patients. We conducted a phase 2b, double-blind, placebo-controlled trial, in which 704 participants (448 male, 256 female) with kidney failure undergoing hemodialysis were randomized to receive lower- or higher-dose osocimab or placebo. In total, 686 participants (436 male, 250 female) received treatment for ≤18 months (planned minimal treatment period of 6 months). The co-primary outcomes were clinically relevant bleeding (a composite of major and clinically relevant nonmajor bleeding) and a composite of the incidence of moderate, severe or serious adverse events. Clinically relevant bleeding occurred in 16/232 (6.9%) and 11/224 (4.9%) participants who received lower- and higher-dose osocimab, respectively, and in 18/230 participants (7.8%) who received a placebo. For the composite adverse event endpoint, incidences were 51%, 47% and 43% in the lower-dose osocimab, higher-dose osocimab and placebo groups, respectively. These results suggest that osocimab is associated with a low risk of bleeding and is generally well tolerated in this population; findings that require confirmation in larger trials. ClinicalTrials.gov identifier, NCT04523220 .
© 2024. The Author(s).
Conflict of interest statement
L.B.T., J.H., Á.F.P. and D.K. are employees of Bayer; J.I.W. holds the Canada Research Chair (Tier I) in Thrombosis and the Heart and Stroke Foundation J.F. Mustard Chair in Cardiovascular Research at McMaster University, and has served as a consultant and received honoraria from Alnylam, Alexion, Anthos, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Janssen, Merck, PhaseBio, Regeneron, Servier and VarmX; J.F. reports consultancy or speaker honoraria from Alnylam, AstraZeneca, Bayer, Boehringer Ingelheim, Calliditas, Chinook, Novartis, Omeros, Travere, VeraTx and Vifor; he also serves on data safety monitoring boards of trials by Novo Nordisk and Visterra. J.F. is an associate editor of
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

