Liposomal Formulation Reduces Transport and Cell Uptake of Colistin in Human Lung Epithelial Calu-3 Cell and 3D Human Lung Primary Tissue Models
- PMID: 38366100
- PMCID: PMC11575476
- DOI: 10.1208/s12249-024-02753-6
Liposomal Formulation Reduces Transport and Cell Uptake of Colistin in Human Lung Epithelial Calu-3 Cell and 3D Human Lung Primary Tissue Models
Abstract
Respiratory tract infections caused by multi-drug-resistant (MDR) bacteria have been a severe risk to human health. Colistin is often used to treat the MDR Gram-negative bacterial infections as a last-line therapy. Inhaled colistin can achieve a high concentration in the lung but none of aerosolized colistin products has been approved in the USA. Liposome has been reported as an advantageous formulation strategy for antibiotics due to its controlled release profile and biocompatibility. We have developed colistin liposomal formulations in our previous study. In the present study, the cellular uptake and transport of colistin in colistin liposomes were examined in two human lung epithelium in vitro models, Calu-3 monolayer and EpiAirway 3D tissue models. In both models, cellular uptake (p < 0.05) and cellular transport (p < 0.01) of colistin were significantly reduced by the colistin liposome compared to the colistin solution. Our findings indicate that inhaled colistin liposomes could be a promising treatment for extracellular bacterial lung infections caused by MDR Pseudomonas aeruginosa (P. aeruginosa).
Keywords: 3D human lung epithelial tissue model; Calu-3 cell; colistin; liposome; pulmonary delivery.
© 2024. The Author(s), under exclusive licence to American Association of Pharmaceutical Scientists.
Figures

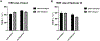

References
-
- Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. The Lancet Infectious Diseases. 2006;6(9):589–601. - PubMed
-
- Grégoire N, Aranzana-Climent V, Magréault S, Marchand S, Couet W. Clinical Pharmacokinetics and Pharmacodynamics of Colistin. Clinical Pharmacokinetics. 2017;56(12):1441–60. - PubMed
-
- Beringer P The clinical use of colistin in patients with cystic fibrosis. Current Opinion in Pulmonary Medicine. 2001;7(6):434–40. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

