Development of antifungal fibrous ocular insert using freeze-drying technique
- PMID: 38366116
- PMCID: PMC11291584
- DOI: 10.1007/s13346-024-01527-8
Development of antifungal fibrous ocular insert using freeze-drying technique
Abstract
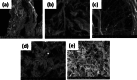
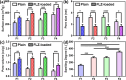
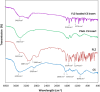
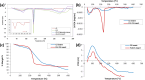
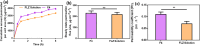
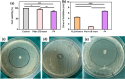
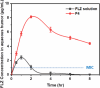
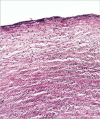
Candida species is one of the pathogenic fungi of the eye responsible for keratitis that frequently causes vision impairment and blindness. Effective treatment requires long-term use of antifungal drugs, which is opposed by the defensive mechanisms of the eye and inadequate corneal penetration. The objective of this study was to develop a carrier for prolonged ocular application of fluconazole (FLZ) to treat keratitis. FLZ was encapsulated into chitosan fibrous matrices (F1-F4) using different chitosan concentrations (0.02, 0.1, 0.5, and 1%w/v, respectively) by freeze-drying as a single-step technique. Studying the morphology and surface properties of the inserts revealed a porous matrix with fibrous features with a large surface area. Thermal stability and chemical compatibility were confirmed by DSC/TGA/DTA and FT-IR, respectively. Loading capacity (LC) and entrapment efficiency (EE) were determined. According to the in vitro release study, F4 (0.11 mg mg-1 LC and 87.53% EE) was selected as the optimum insert because it had the most sustained release, with 15.85% burst release followed by 75.62% release within 12 h. Ex vivo corneal permeation study revealed a 1.2-fold increase in FLZ permeation from F4 compared to FLZ aqueous solution. Also, in the in vivo pharmacokinetic study in rabbits, F4 increased the AUC0-8 of FLZ by 9.3-fold and its concentration in aqueous humor was maintained above the MIC through the experimentation time. Studies on cytotoxicity (MTT assay) provide evidence for the safety and biocompatibility of F4. Therefore, the freeze-dried FLZ-loaded chitosan fibrous insert could be a promising candidate for treating ocular keratitis.
Keywords: Candida; Chitosan; Fluconazole; Freeze-drying; Insert; Ocular delivery.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Qian L, Zhang H. Green synthesis of chitosan-based nanofibers and their applications. Green Chem. 2010;12:1207–14. 10.1039/b927125b.10.1039/b927125b - DOI
-
- Roesky C. Overcoming the challenges of ophthalmic delivery using aqueous-free technology: redefining dry eye disease. ONdrugDelivery. 2018;82:10–4.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

