Synthetic modified vaccinia Ankara vaccines confer cross-reactive and protective immunity against mpox virus
- PMID: 38366141
- PMCID: PMC10873322
- DOI: 10.1038/s43856-024-00443-9
Synthetic modified vaccinia Ankara vaccines confer cross-reactive and protective immunity against mpox virus
Abstract
Background: Although the mpox global health emergency caused by mpox virus (MPXV) clade IIb.1 has ended, mpox cases are still reported due to low vaccination coverage and waning immunity. COH04S1 is a clinically evaluated, multiantigen COVID-19 vaccine candidate built on a fully synthetic platform of the highly attenuated modified vaccinia Ankara (MVA) vector, representing the only FDA-approved smallpox/mpox vaccine JYNNEOS. Given the potential threat of MPXV resurgence and need for vaccine alternatives, we aimed to assess the capacity COH04S1 and its synthetic MVA (sMVA) backbone to confer MPXV-specific immunity.
Methods: We evaluated orthopoxvirus-specific and MPXV cross-reactive immune responses in samples collected during a Phase 1 clinical trial of COH04S1 and in non-human primates (NHP) vaccinated with COH04S1 or its sMVA backbone. MPXV cross-reactive immune responses in COH04S1-vaccinated healthy adults were compared to responses measured in healthy subjects vaccinated with JYNNEOS. Additionally, we evaluated the protective efficacy of COH04S1 and sMVA against mpox in mpox-susceptible CAST/EiJ mice.
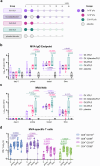
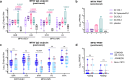
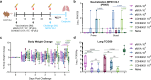
Results: COH04S1-vaccinated individuals develop robust orthopoxvirus-specific humoral and cellular responses, including cross-reactive antibodies to MPXV-specific virion proteins as well as MPXV cross-neutralizing antibodies in 45% of the subjects. In addition, NHP vaccinated with COH04S1 or sMVA show similar MPXV cross-reactive antibody responses. Moreover, MPXV cross-reactive humoral responses elicited by COH04S1 are comparable to those measured in JYNNEOS-vaccinated subjects. Finally, we show that mice vaccinated with COH04S1 or sMVA are protected from lung infection following challenge with MPXV clade IIb.1.
Conclusions: These results demonstrate the capacity of sMVA vaccines to elicit cross-reactive and protective orthopox-specific immunity against MPXV, suggesting that COH04S1 and sMVA could be developed as bivalent or monovalent mpox vaccine alternatives against MPXV.
Plain language summary
Mpox is an ilness caused by the mpox virus (MPXV) that belongs to the poxvirus family. The 2022-2023 mpox outbreak highlights the need to develop effective vaccines against MPXV. We have developed a COVID-19 vaccine using as scaffold chemically synthesized genetic material of a highly attenuated and safe poxvirus vector. This scaffold is the same present in a vaccine that has been approved and is given to prevent mpox. Here, we show that healthy human volunteers or monkeys vaccinated with this COVID-19 vaccine generated a robust immune response against MPXV, similar to that generated by the mpox vaccine with the same scaffold. This COVID-19 vaccine is also able to protect mice from infection caused by the MPXV strain isolated from the recent mpox outbreak. This COVID-19 vaccine in a poxvirus scaffold might be an additional tool to curtail mpox outbreaks.
© 2024. The Author(s).
Conflict of interest statement
While unknown whether publication of this report will aid in receiving grants and contracts, it is possible that this publication will be of benefit to City of Hope (COH). COH had no role in the conceptualization, design, data collection, analysis, decision to publish, or preparation of the manuscript. The authors declare the following competing interests: D.J.D. and F.W. are co-inventors on a patent application covering the design and construction of the synthetic MVA platform (PCT/US2021/016247). D.J.D., F.W., and F.C. are co-inventors on a patent application covering the development of a COVID-19 vaccine (PCT/US2021/032821) and provisional applications covering MPXV. D.J.D. is a consultant for GeoVax Labs and Helocyte Inc. A.F. is a consultant for Pfizer. A.S. is a consultant for Consultant for AstraZeneca Pharmaceuticals, Calyptus Pharmaceuticals, Inc, Darwin Health, EmerVax, EUROIMMUN, F. Hoffman-La Roche Ltd, Fortress Biotech, Gilead Sciences, Gritstone Oncology, Guggenheim Securities, Moderna, Pfizer, RiverVest Venture Partners, and Turnstone Biologics. All other authors declare no competing interests. GeoVax Labs Inc. has taken a worldwide exclusive license for COH04S1 under the name of GEO-CM04S1.
Figures
References
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

