Inhibition of SFTSV replication in humanized mice by a subcutaneously administered anti-PD1 nanobody
- PMID: 38366162
- PMCID: PMC10940662
- DOI: 10.1038/s44321-024-00026-0
Inhibition of SFTSV replication in humanized mice by a subcutaneously administered anti-PD1 nanobody
Abstract
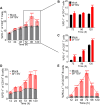
Severe fever with thrombocytopenia syndrome (SFTS) is a life-threatening disease caused by a novel bunyavirus (SFTSV), mainly transmitted by ticks. With no effective therapies or vaccines available, understanding the disease's mechanisms is crucial. Recent studies found increased expression of programmed cell death-1 (PD-1) on dysfunctional T cells in SFTS patients. However, the role of the PD-1/programmed cell death-ligand 1 (PD-L1) pathway in SFTS progression remains unclear. We investigated PD-1 blockade as a potential therapeutic strategy against SFTSV replication. Our study analyzed clinical samples and performed in vitro experiments, revealing elevated PD-1/PD-L1 expression in various immune cells following SFTSV infection. An anti-PD-1 nanobody, NbP45, effectively inhibited SFTSV infection in peripheral blood mononuclear cells (PBMCs), potentially achieved through the mitigation of apoptosis and the augmentation of T lymphocyte proliferation. Intriguingly, subcutaneous administration of NbP45 showed superior efficacy compared to a licensed anti-PD-1 antibody in an SFTSV-infected humanized mouse model. These findings highlight the involvement of the PD-1/PD-L1 pathway during acute SFTSV infection and suggest its potential as a host target for immunotherapy interventions against SFTSV infection.
Keywords: Nanobody; NbP45; PD-1 Blockade; SFTSV; Subcutaneous Injection.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





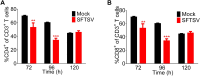

References
-
- Bekerman E, Hesselgesser J, Carr B, Nagel M, Hung M, Wang A, Stapleton L, von Gegerfelt A, Elyard HA, Lifson JD, et al. PD-1 blockade and TLR7 activation lack therapeutic benefit in chronic simian immunodeficiency virus-infected macaques on antiretroviral therapy. Antimicrob Agents Chemother. 2019;63:e01163–19. - PMC - PubMed
-
- Chen C, Li P, Li K-F, Wang H-L, Dai Y-X, Cheng X, Yan J-B. Animals as amplification hosts in the spread of severe fever with thrombocytopenia syndrome virus: a systematic review and meta-analysis. Int J Infect Dis. 2019;79:77–84. - PubMed
-
- Choi S, Kim M-C, Kwon J-S, Kim J-Y, Lee KH, Kim S-H. Case Report: use of plasma exchange followed by convalescent plasma therapy in a critically Ill patient with severe fever and thrombocytopenia syndrome-associated encephalopathy: cytokine/chemokine concentrations, viral loads, and antibody responses. Am J Trop Med Hyg. 2018;99:1466–1468. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 32370988/MOST | National Natural Science Foundation of China (NSFC)
- 31970149/MOST | National Natural Science Foundation of China (NSFC)
- U22A20335/MOST | National Natural Science Foundation of China (NSFC)
- 2018ZX10301406/The Major Research and Development Project
- Grant# 2017BN04/Nanjing University-Ningxia University Collaborative Project
LinkOut - more resources
Full Text Sources
Research Materials

